Introduction
Superior vena cava syndrome (SVCS) is a clinical condition characterized by a constellation of signs and symptoms resulting from the obstruction of blood flow through the superior vena cava (SVC) 1 . This syndrome is most commonly associated with malignant tumors, which account for approximately 60% of cases, while iatrogenic causes, such as thrombosis or stenosis induced by central lines or medical devices, contribute to 30-40% of cases 2 . The incidence of SVCS has been rising, correlating with the increasing utilization of catheters, pacemakers, and implantable cardioverter defibrillators (ICDs) 3 .
Anatomically, the SVC is responsible for draining approximately one-third of the venous blood into the heart, receiving venous return from the right and left brachiocephalic veins, which in turn drain the head and upper extremities. On computed tomography (CT) imaging, the SVC typically measures 7.1 ± 1.4 cm in length with a diameter of 2.1 ± 0.7 cm, though these measurements can vary depending on blood volume. A cross-sectional diameter of less than 1.07 cm² on CT is indicative of SVC obstruction or compression 3 .
Clinically, SVCS often presents with facial and hand swelling, distension of subcutaneous vessels, cyanosis, and plethora. Respiratory symptoms, including dyspnea, stridor, cough, hoarseness, and dysphagia, arise due to edema and swelling in the 4 . Additionally, cardiac function may be compromised due to the mass effect on the heart or impaired venous return caused by SVC obstruction. These symptoms typically exacerbate in the supine position. Moreover, specific signs and symptoms may vary depending on the underlying etiology of the SVCS 5 .
Case Report
A 54-year-old male presented to the Emergency Department (ED) with complaints of syncope and breathlessness persisting for the past two weeks, accompanied by fever. The patient also reported a history of voice loss over the preceding two months and a significant decrease in food intake.
Upon arrival, the primary survey revealed a patent airway, but the trachea was deviated to the left. He was tachypneic with a respiratory rate of 28 cycles per minute and hypoxic with an oxygen saturation of 85% on room air. Oxygen therapy was initiated with a non-rebreather mask (NRBM) at 15 liters per minute, resulting in an improved saturation of 95% in a propped-up position. Jugular venous pressure (JVP) was elevated to 12 cm, and auscultation revealed bilateral basal crepitation. He was tachycardic with a pulse of 130 beats per minute, blood pressure of 100/60 mm Hg, and a capillary refill time (CRT) of less than 3 seconds. His Glasgow Coma Scale (GCS) was 15/15 (E4V5M6), with normal higher mental functions and bilaterally equal and reactive pupils (3 mm). Random blood glucose levels were 102 mg/dl. On exposure, he was noted to have multiple engorged veins all over the chest and abdomen (Figure 1).

Adjuncts used during the primary survey included arterial blood gas (ABG), which indicated metabolic acidosis with compensation, a chest radiograph showing lung hyperinflation, leftward tracheal deviation, increased parasternal space, and prominent broncho-vascular markings (Figure 2), and electrocardiography (ECG) demonstrating sinus tachycardia.
During the secondary survey, it was discovered that the patient had a history of Chronic Obstructive Pulmonary Disease (COPD) and hypertension, managed with a metered-dose inhaler (MDI) containing Budesonide and Formoterol (200mcg + 6mcg) and tablet Amlodipine 5 mg. He had a long-standing history of smoking, consuming 2-3 packs of beedis (tobacco rolled in leaf) per day for over 25 years. The patient reported progressive difficulty in speech and loss of appetite over the past month, followed by breathlessness and fever for the last two weeks, which worsened in a supine position and was relieved by propping up.
Given the clinical presentation, a suspicion of superior vena cava (SVC) obstruction secondary to a possible mediastinal mass, along with COPD and systemic hypertension, was raised.

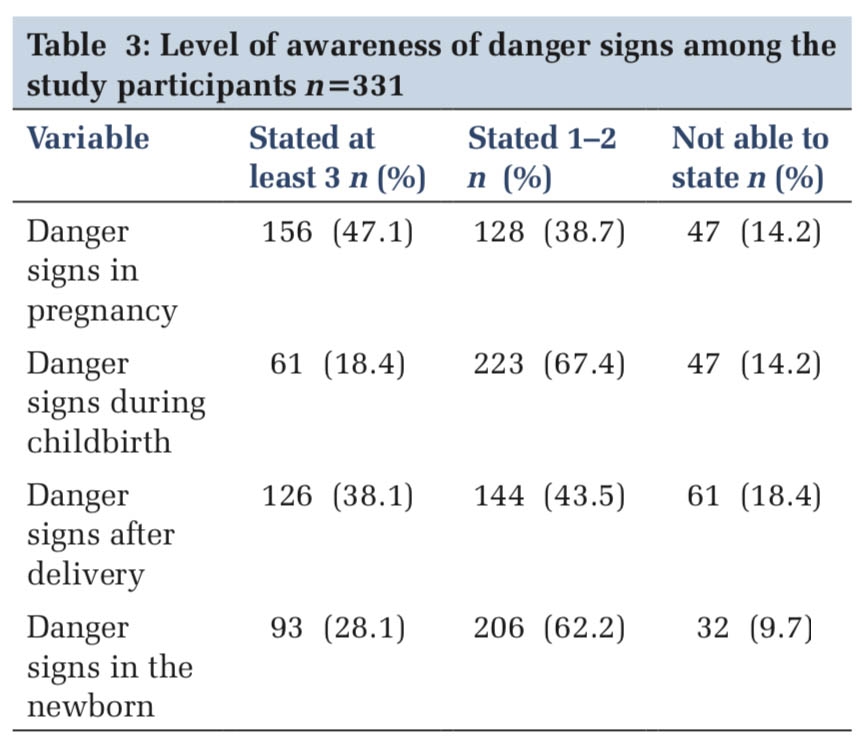
In the ED, the patient was managed in a propped-up position and continued on 15 liters of oxygen via NRBM. Nebulization with ipratropium bromide, levosalbutamol, and budesonide was administered, along with intravenous corticosteroids (Hydrocortisone), antiemetics (Ondansetron), and proton pump inhibitors (Pantoprazole). Base line blood investigations, including a complete hemogram, liver function test, renal function test, and erythrocyte sedimentation rate, were obtained (Table 1).
|
Sl. No |
Test Name |
Results |
Units |
Normal Range |
|
1. |
Complete Hemogram |
|||
|
|
Hb |
13.30 |
gm% |
14.50-15.50 |
|
|
WBC |
13,930 |
cells/cumn |
4,000-11,000 |
|
|
Platelets |
2.20 |
lakh/cumn |
1.50-4.50 |
|
|
Neutrophils |
80.60 |
% |
40.00-75.00 |
|
2. |
Arterial Blood Gas Analysis |
|||
|
|
pH |
7.2 |
|
7.35-7.45 |
|
|
PCO2 |
32.1 |
mm Hg |
35-45 |
|
|
PO2 |
178 |
mm Hg |
85-100 |
|
|
SO2 |
89 |
% |
93-98 |
|
|
HCO3 |
16 |
mmol/L |
22-28 |
|
|
Lactate |
4.6 |
mmol/L |
<2 |
|
3. |
Renal Function Test |
|||
|
|
Urea |
36.90 |
mg/dl |
16.6-48.5 |
|
|
Uric acid |
6 |
mg/dl |
Male: 3.4-7.0 Female: 2.4-5.7 |
|
|
Serum creatinine |
1.9 |
mg/dl |
0.50-0.90 |
|
4. |
Liver Function Test |
|||
|
|
AST |
32 |
U/L |
5-30 |
|
|
ALT |
28 |
U/L |
4-36 |
|
|
Albumin |
4.8 |
gm/dl |
35-50 |
|
|
Globulin |
16 |
gm/dl |
25-35 |
|
|
Total protein |
7.2 |
gm/dl |
60-83 |
|
|
ALP |
50 |
IU/L |
4 to 36 IU/L |
|
5. |
Serum Electrolyts |
|||
|
|
Sodium |
140 |
mEq/L |
135.00-145.00 |
|
|
Potassium |
3.90 |
mEq/L |
3.50-5.10 |
|
|
Chloride |
106 |
mEq/L |
98.00-111.00 |
|
6. |
ESR |
17.00 |
mm/hr |
0.00-15.00 |
|
7. |
Troponin I |
<0.1 |
ng/ml |
0.1-0.3 |
Hb - Haemoglobin, WBC - White blood cells, AST - Aspartate aminotransferase, ALT - Alanine transaminase, ALP - Alkaline phosphatase, ESR - Erythrocyte sedimentation rate, PCO2 - Partial Pressure of Carbon Di-Oxide, PO2 - Partial of Oxygen, SO2 - Oxygen Saturation
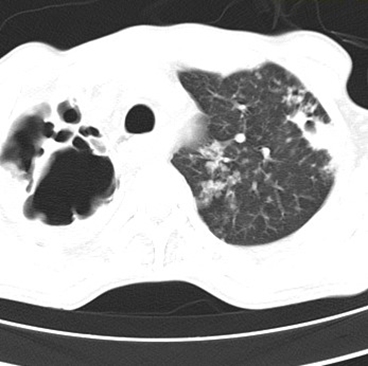
A contrast-enhanced computed tomography (CECT) of the neck and thorax revealed multiple heterogeneously enhancing conglomerated mediastinal nodes with necrotic areas in the bilateral supraclavicular, bilateral upper and lower paratracheal, perivascular, para-aortic, subcarinal, and paraesophageal regions, measuring 14.7 × 9.3 × 7.6 cm with compression of the trachea and esophagus, encasement of mediastinal vascular structures, and severe compression of the SVC, leading to thoracic collaterals on the right side, suggestive of SVC syndrome. Additionally, tiny nodular opacities were observed in the anterior segment of the right upper lobe (4.7 × 7.7 mm) and subpleural opacities in the superior segment of the left lower lobe (3 × 3 mm). Emphysematous changes in both lungs, mild bilateral pleural effusion, and adjacent atelectasis were also noted (Figure 3 a, b, c).

A definitive diagnosis of SVC obstruction secondary to a compressing tumor, likely of metastatic origin, was established. The patient was subsequently transferred to critical care, where a true-cut biopsy was planned after stabilizing the patient's hemodynamic status. The biopsy, performed a week later, suggested a possible lymphoma. The patient was later shifted to ward after 5 days.
Given the poor prognosis associated with surgical intervention, the patient was scheduled for chemotherapy using the R-CHOP regimen, consisting of rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone. The patient was followed up in the oncology.
Discussion
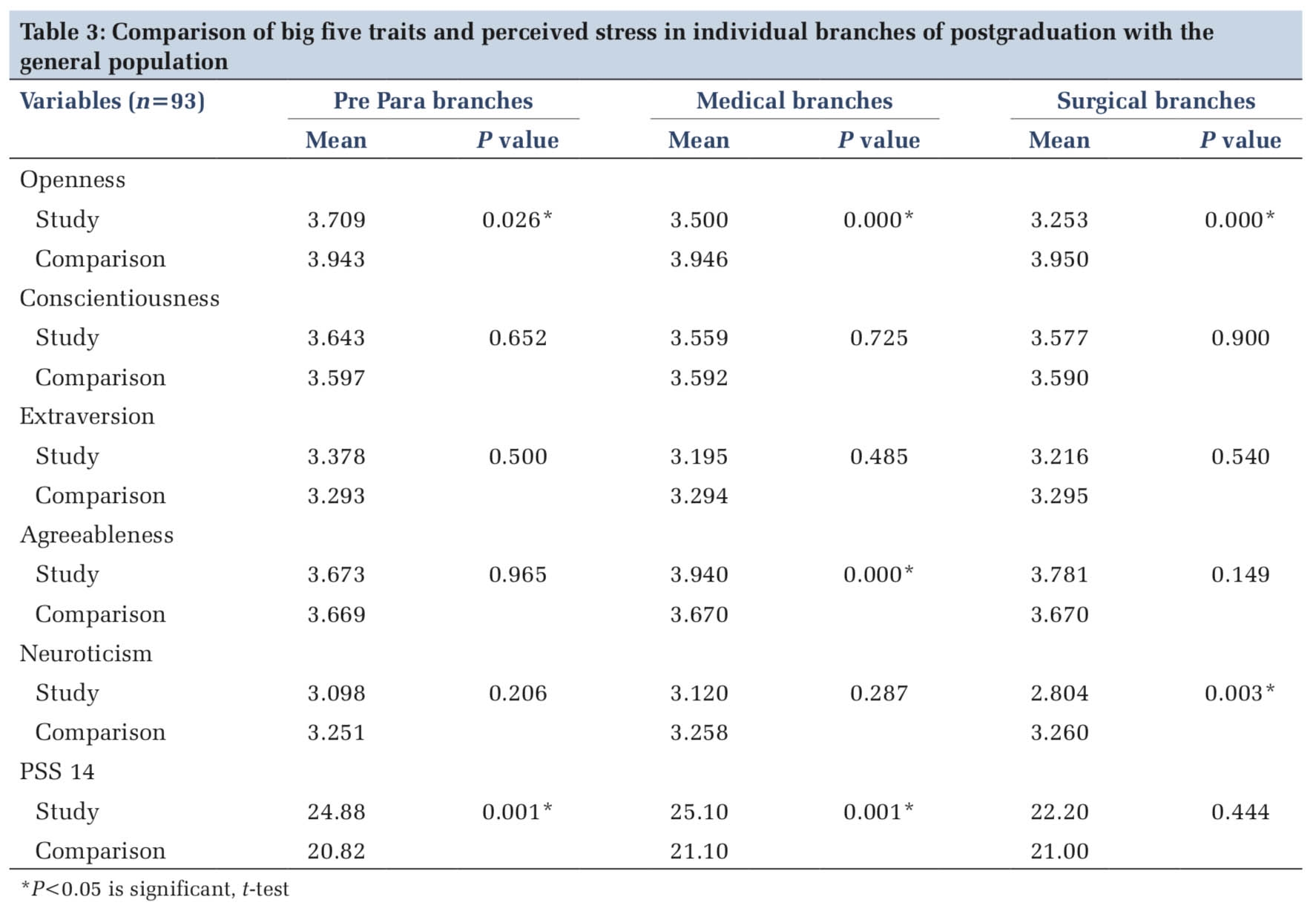
Superior vena cava syndrome (SVCS) remains a critical condition primarily associated with malignant etiologies. Its clinical manifestations can vary significantly, ranging from mild symptoms to severe life-threatening presentations. The intricate relationship between the underlying cause and the severity of symptoms necessitates a nuanced approach to diagnosis and management. While the majority of SVCS cases are linked to malignancies, a comprehensive understanding of both malignant and benign causes is essential for guiding treatment strategies 6 .
This case highlights the atypical presentation of malignant SVCS as syncope upon arrival at the emergency department, underscoring the crucial importance of accurate diagnosis, grading, and staging in the effective management of this condition 1 . Our patient’s SVCS was secondary to lymphoma, which, although less common than other malignancies, demonstrates the diverse etiologies of SVCS. Typically, non-small cell lung cancer is the predominant malignancy associated with SVCS, accounting for approximately 50% of cases. Other malignant causes include small cell lung cancer, malignant lymphomas, germ cell tumors, malignant mesotheliomas, and thymic tumors 2, 7 .
While malignant causes dominate, benign etiologies are responsible for around 30% of SVCS cases. Among these, device-related factors such as central venous catheters, pacemakers, defibrillators, and indwelling hemodialysis catheters are particularly notable, contributing to approximately 25% to 30% of benign SVCS cases. Other benign causes include radiation fibrosis, infections, retrosternal thyroid enlargement, aortic aneurysms, benign tumors, mediastinal hematomas, and sarcoidosis 3, 4 . It is rare for SVCS to be directly associated with chronic obstructive pulmonary disease (COPD) or emphysema, though some cases have linked SVCS to the compression of the superior vena cava by giant bullous lesions 8 .
The clinical manifestations of SVCS are typically related to the gradual obstruction of the superior vena cava, allowing for the development of collateral circulation. Common symptoms include swelling of the neck, upper extremities, and face, along with dyspnea, chest pain, cough, weight loss, jugular venous distension, and dilated collateral chest veins. The gradual onset of symptoms, as observed in our patient, aligns with the typical presentation of SVCS, where symptoms develop over time 3, 9 .
This case is particularly notable for the absence of prominent neck and upper extremity edema, despite the presence of dilated collateral chest veins, which may indicate a slow progression of lung hyperinflation. The radiographic findings, such as the drop-like appearance of the heart and the flattened diaphragm, are well-established indicators of lung hyperinflation. However, it is important to recognize that lung hyperinflation does not invariably lead to SVCS 5, 6 .
The anatomical positioning of the superior vena cava is a critical factor in its vulnerability to compression. The SVC is typically located in the upper mediastinum on the right side, and in some cases, it may extend into the lung field. When this occurs, the SVC becomes more susceptible to compression, particularly in the absence of protective mediastinal adipose tissue. In individuals with COPD, the SVC’s extension into the lung field may contribute to its narrowing, even in the absence of giant bullae, thereby leading to the development of SVCS 8, 10 .
Case reports further illustrate the diverse presentations and etiologies of SVCS. For instances Hassan et al. (Los Angeles, 2016) documented a case of SVCS in a patient who presented with diffuse right upper extremity swelling and distended superficial veins, later determined to be secondary to malignancy 5 . Similarly, Haider et al. (New York, 2022) reported a case where a patient with multiple comorbidities, including pulmonary embolism and deep venous thrombosis, presented with chest pain and upper extremity swelling. In this case, the SVCS was found to be due to thrombosis rather than malignancy 1.
Despite the availability of various treatment options for SVCS, including stenting, endobronchial valve placement, and surgical interventions, these were not pursued in our patient’s case. This decision highlights the complexity of managing SVCS, particularly in cases where lung hyperinflation and anatomical variations contribute to the condition. The findings in this case underscore the importance of considering COPD as a potential contributing factor in SVCS, particularly in patients with atypical presentations and complex clinical histories.
Conclusion
Superior vena cava syndrome results from obstruction of blood flow through the SVC. We encountered a rare oncological emergency, a SVCS secondary to a malignant mass, with a key clue of breathlessness and engorged veins at presentation and associated chronic obstructive pulmonary disease. SVCS should be included in the differential diagnosis for emergency clinicians encountering such presentations. The management of SVCS in the ED poses significant challenges, particularly when complicated by malignancy and associated conditions. This case highlights the necessity of a multidisciplinary approach to effectively address the complexities of oncological emergencies. Prompt and accurate diagnosis, along with coordinated care, is essential for improving patient outcomes in such critical situations.