Introduction
Onychomycosis comprises all fungal infections affecting the nail apparatus, which includes nail matrix, nail plate, cuticle, mesenchymal tissue, and nail folds. Onychomycosis caused by dermatophytes is called tinea unguium.[1] Otheraccountablespeciesforonychomycosisare yeast and yeast-like fungi (i.e., Candida spp., Geotrichum candidum, and Trichosporon beigelii) and saprophytic fungi such as Aspergillus, Scopulariopsis, Fusarium, Acremonium, and Penicillium.[2]
Dermatophytes are closely related fungi that use keratin as a nitrogen source that affects hair, skin, and nail. Three anamorphic genera are Epidermophyton, Microsporum, and Trichophyton.[3] The variations in the presentation are due to species involved, size of the inoculum, involved sites, and host immune status.[4] The resistance of the host is as important as the virulence of the fungi.[3]
The dermatophytes causing onychomycosis are predominantly anthropophilic fungi such as Trichophyton rubrum and Trichophyton mentagrophytes. Epidermophyton floccosum has occasionally been described as an agent causing tinea unguium, generally in association with skin infections. The Microsporum genus is also isolated with much less frequency, including such species as the geophilic Microsporum gypseum, zoophilic M. canis, and anthropophilic M. audouinii.[5] A very low incidence of < 1% of Microsporum canis has been reported from an extensive scale survey done in Paris and Brazil.[5,6]
This case report describes the rarity of dual infections in a young female patient caused by M. gypseum and T. mentagrophytes.
Case Report
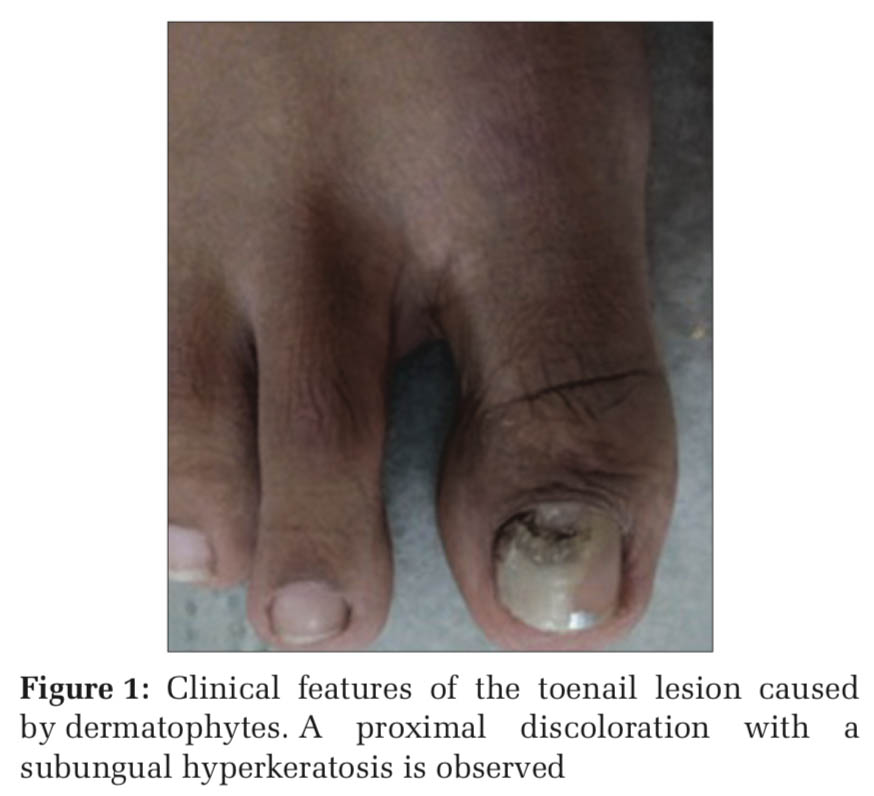
A 20-year-old female from coastal Karnataka with good hygiene practices and in good general health, non-diabetic, and non-smoker, with a history of usage of nail polish regularly, came to dermatology OPD with abnormal discoloration of the right great toe in January 2020. The patient was not on any antibiotics, steroids, or immunosuppressive drugs. On examination, there was proximal nail infection suggestive of onychomycosis. Clinically, the pattern of invasion presented as proximal subungual onychomycosis (PSO), as shown in Figure 1.
The proximal nail scrapings were collected after thoroughly cleaning the affected great toenail with 70% ethyl alcohol and sent to the microbiology lab in a clean paper for fungal culture and KOH mount.
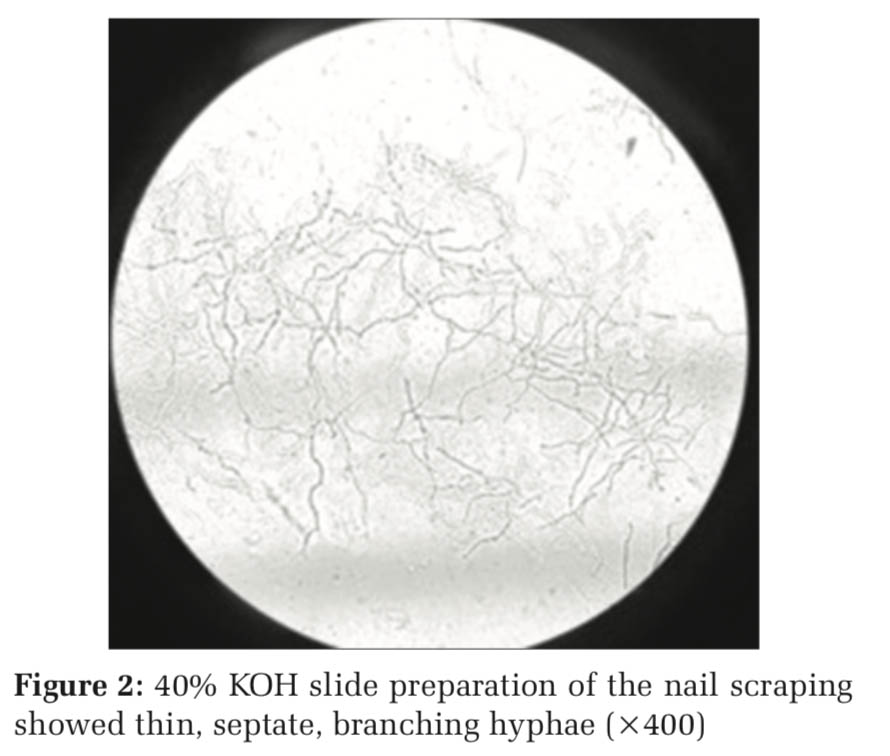
On receiving the nail sample, one portion of the scrapings was put on a clean glass slide, in a drop of a 40% KOH solution, then covered with a clean coverslip and left for 2 h in a moist chamber for the keratin to dissolve. On examination, abundant thin segmented branching hyphae were seen suggestive of dermatophyte infection, as shown in Figure 2.
Further, the remaining portion of scrapings was inoculated on to the middle portion of the Sabouraud’s dextrose agar (SDA) with chloramphenicol tubes in the BOD incubator and room temperature. After a week of inoculation, the fungal growth appeared with two types of fungus in both the tubes. They were subcultured in two SDA tubes and simultaneously inoculated to the dermatophyte test medium.[3,4]
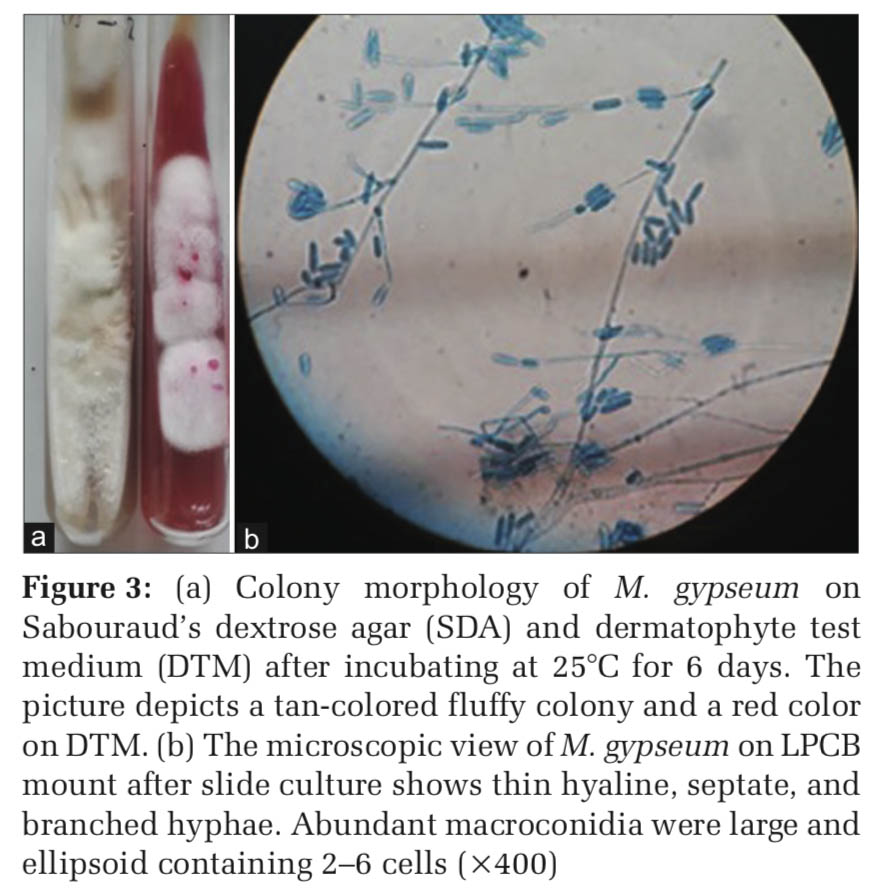
The first isolate from the SDA tube on macroscopic examination showed tan-colored, granular to the fluffy colony with no pigment on the reverse. On DTM, the media turned from yellow to red-colored. On LPCB tease mount and slide culture, thin hyaline, septate, and branched hyphae seen. Abundant macroconidia were large and ellipsoid containing 2–6 cells, as shown in Figure 3. Occasional clavate microconidia were appreciated. The hair perforation test was performed, showing perforation. This isolate was identified as M. gypseum, a geophilic fungus.
The second isolate on macroscopy showed a flat, granular white colony on the obverse with yellow pigment on the reverse. On LPCB mount and slide culture hyaline, septate, spiral, and branched hyphae seen. Abundant pyriform shaped microconidia singly and in clusters seen. Few rat tails like macroconidia seen on the hyphae. Urease test was hydrolyzed, turning the media into a pink color in two days, as shown in Figure 4. The penetration of hair was observed in vitro. The fungus identified as T. mentagrophytes.
Remarkably, treatment was immediately started according to the direct KOH mount examination results and terbinafine was prescribed in an oral dosage of 250 mg daily for six weeks. The patient showed considerable outcome to the treatment, as shown in Figure 5.
Discussion
Trichophyton rubrum, T. mentagrophytes, and E. floccosum are the most common etiologic agents worldwide, causing onychomycosis.[7] In our case, we observed that the patient had the most common and well-established etiological agent, that is, T.mentagrophytes causing onychomycosis. Also interestingly, we identified an uncommon etiological agent, M. gypseum, which is generally considered to involve only hair and skin but not nail.
M. gypseum is a soil saprophyte and the source of human infection has been traced to soil, dogs, and cats. In a previous study conducted by Martinez et al., M. gypseum infection presented as Distal Lateral Subungual Onychomycosis (DLSO), reported in young women with no risk factors for infection suggested.[6] While in our case, the presentation was PSO with no risk factors.
There is documented evidence by Szepietowski[8] for the coexistence of toenail onychomycosis with other dermatomycoses, especially tinea pedis. However, in this report, no such coexistence was found. A mixed Chaetomium globosum/T. mentagrophytes onychomycosis, a nondermatophyte and dermatophyte, was reported by Jean et al.[9] This article emphasizes the probability of mixed infections in the case of onychomycosis.
Dual infections of dermatophytes are a possibility; hence, a keen observation of macroscopy helps to rule out contamination or mixed infections. There is a need for a mycologist to have a high index of suspicion in such cases to proceed further in identification till species level for obtaining the exact etiology of onychomycosis, thereby help in better treatment, reducing the discomfort to patients.


|

