

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2018.v04i01.002
Year: 2018, Volume: 4, Issue: 1, Pages: 5-14
Review Article
Deepa Bangalore Gotur
Department of Medicine, Weill Cornell Medical College, Intensivist, Houston Methodist Hospital, Houston, Texas, United States
Delirium is a frame of mind characterized by transient and fluctuating cognitive incapacity and inattention due to a variety of illnesses. Intensive care unit (ICU)-delirium can present as hypoactive, hyperactive or mixed delirium types, hypoactive being the most common and often under-recognized. Prevalence of ICU-delirium is variable based on type of patient population but could be as high as 77%. Recent theory for the development of delirium is known as the systems integration failure hypothesis. Predisposing risk factors for ICU delirium are advanced age, baseline cognitive impairment, increased comorbid disease, frailty, alcohol and drug abuse, and high severity of illness. The ABCDEF bundle is a group of interventions developed by the Society of Critical Care Medicine that when applied collectively can help reduce delirium, improve pain management and reduce long-term consequences for adult ICU patients. Delirium outcomes range from mortality, variable degree of cognitive and functional deficits to full functional recovery.
KEY WORDS: Delirium, intensive care unit delirium, confusion assessment method- intensive care unit, dexmedetomidine, acute brain failure, hypoactive delirium.
“On the 14th day, Louie was lying beside Phil under the canopy when he abruptly sat up. He could hear singing. He kept listening: It sounded like a choir. He nudged Phil and asked him if he heard anything ... Above him, floating in a bright cloud, he saw human figures, silhouetted against the sky. He counted 21 of them. They were singing the sweetest song he had ever heard ... Phil had heard and seen nothing. Whatever this had been, Louie concluded, it belonged to him alone” goes the heroic story of Louis Zamperini lost in the Pacific Ocean for a harrowing 47 days surviving sharks, storms, starvation, dehydration, and delirium during World War II as narrated in the book, “Unbroken” by Laura Hillenbrand.[1] Delirium, as exemplified in this true story, is a serious disturbance in mental abilities and is widespread and under diagnosed. The ability of the intensive care unit (ICU) to sustain, support and even replace organ functions is exponentially growing, and the core capability of using invasive techniques underpins the practice of critical care medicine, leaving critically ill patients vulnerable to ICU acquired delirium. Here, we review prevention and management with a focus on ICU related delirium (ICU-Delirium).
Delirium is a frame of mind characterized by transient and fluctuating cognitive incapacity and inattention due to a variety of illnesses. Five main characteristics of this disorder are cognitive impairment, attention deficits, circadian rhythm dysregulation, emotional lability, and alteration in psychomotor functioning.[2] It can present as hypoactive, hyperactive or mixed delirium types, hypoactive being the most common (65%) and often under-recognized.[3] The two international classifications - International Classification of Diseases and Related Health Problems, 10th Revision from the World Health Organization (ICD-10) and Diagnostic and Statistical Manual of Mental Disorders, from American Psychiatric Association (DSM), define delirium as a disorder. DSM-V defines delirium as the following:
A. Disturbance in attention (i.e., reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment)
B. Disturbance develops over a short period, represents an acute change from baseline attention and awareness, and tends to fluctuate in severity during the course of the day
C. Disturbance in cognition (i.e., memory deficit, disorientation, language, visuospatial ability, or perception)
D. Disturbances are not better explained by a pre- existing, established or evolving neurocognitive disorder and do not occur on the context of a severely reduced level of arousal such as coma
E. There is evidence form the history, physical examination, or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal, or exposure to a toxin or due to multiple etiologies.
ICD-10 definition for delirium is an etiologically nonspecific organic cerebral syndrome characterized by concurrent disturbances of consciousness and attention, perception, thinking, memory, psychomotor behavior, emotion, and the sleep-wake schedule. The duration is variable, and the degree of severity ranges from mild to very severe.
In 500 BC, Hippocrates first referred to delirium as phrenitis describing mental abnormalities caused by fever, poisoning, or head trauma. He also used the term lethargus to describe dulling of sense and believed that during the course of illness lethargus can change to phrenitis and vice versa. In first century AD, Celsus introduced the term delirium, a Latin derivative de-lira meaning “to go out of the furrow or deviate from a straight line or deranged.” In the medieval times the historian Procopius while describing possibly the bubonic disease in Constantinople gave an accurate account of the characteristics of delirium as insomnia, excitement, shouting, rushing off in flight, and becoming violent in some; while others drifted to coma, sleeping constantly and dying from lack of food or water. The 19th century came away from the old terminologies, focusing more on psychopathology and prognosis as grounds for definition as well as distinguishing delirium related to alcohol withdrawal as a separate condition. Engel and Romano in 1959 showed using electroencephalogram that delirium was a disturbance in the level of consciousness manifesting as cognitive attentional disturbances and was due to disruption of brain metabolism.[4] In the 1980s Zbigniew J. Lipowski, who worked extensively on delirium is regarded as the father of modern research into this condition.[5]
Delirium as an organ impairment in critically ill patients is under-recognized, and hence, the true incidence of the extent of this problem is underestimated. A single day point-prevalence international study undertaken in 104 ICUs across 11 countries from North and South Americas and Spain found a delirium prevalence of 32.3%.[6] In a systematic review of observational epidemiological studies involving 16,595 patients in 42 studies, the incidence of delirium was found to be 31.8% in critically ill patients.[7] This number could be higher in specialized ICUs; for example, in ventilated burns patients the prevalence is as high as 77%.[8] Acute stroke predisposes to delirium, and the incidence is 10–48%[9] while 24% of trauma patients screened positive for delirium.[10] The incidence of post- operative delirium is 4–53.3%, and the proportion of delirium is higher in hip-fracture ranging from 34% to 92%.[11] The incidence of delirium in emergency departments is about 8.3%. A vast majority is the hypoactive type, and the diagnosis is missed in 76% of cases.[12] In a point prevalence study involving 311 general hospitals, 17.6% or 19.6% of general ward patients had delirium based on whether confusion assessment method (CAM) or DSM-IV criteria were used for diagnosis.[13]
A single center tertiary hospital study in India that used Delirium Rating Scale-Revised-98 (DRS-R-98) scoring system found the prevalence of delirium was high at 53.6%.[14] In a cardiac ICU in India, delirium assessed using CAM-ICU found an incidence rate of 9.27% and a prevalence of 18.77%.[15] In a recent single-center medical/surgical ICU study conducted in Chandigarh, India, the prevalence rate was 68.2%, and they had low referral rates to a psychiatric team for delirium (1.7%).[16]
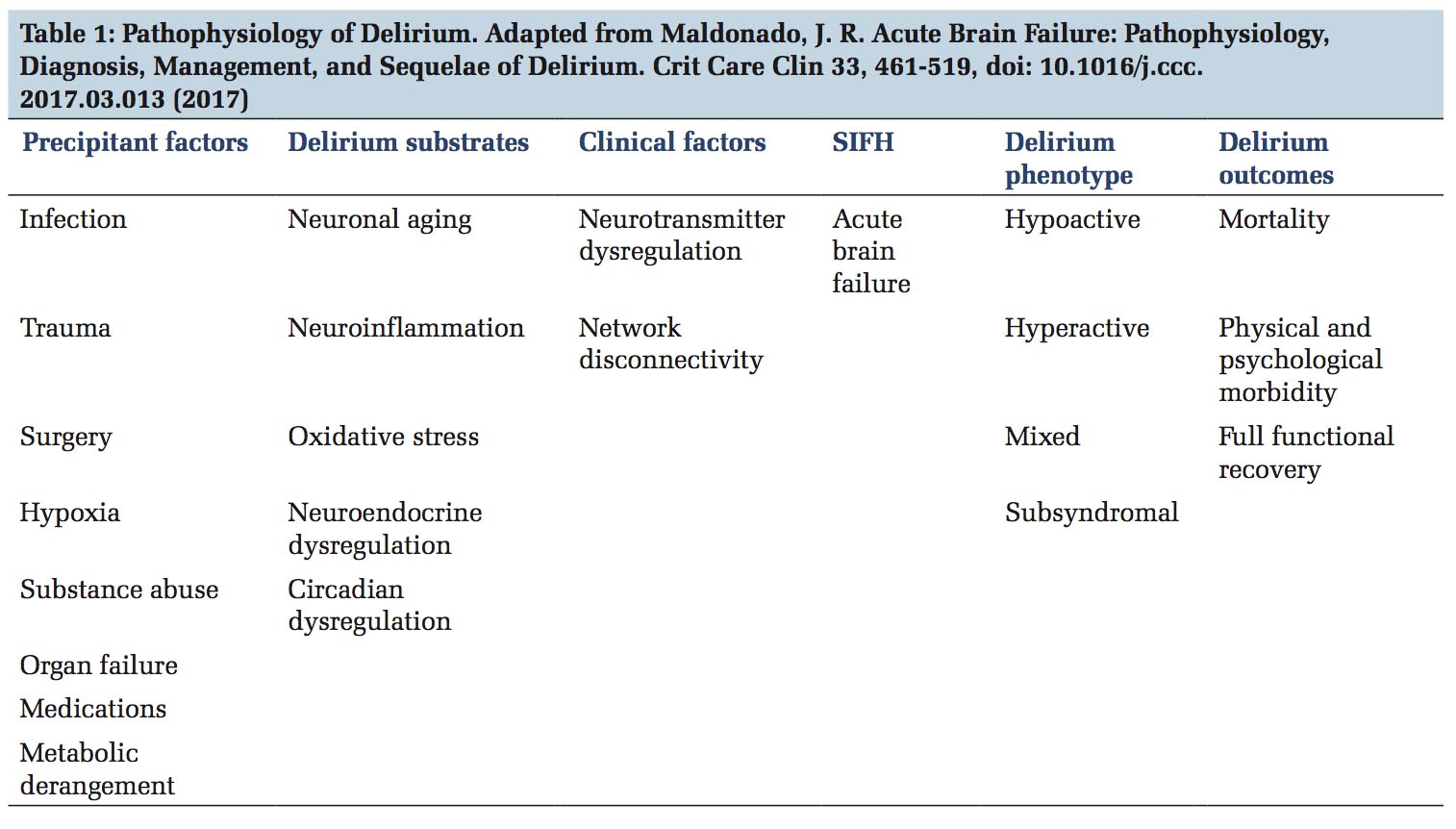
Recent theory for the development of delirium is known as the systems integration failure hypothesis. This hypothesis integrates precipitant factors, delirium substrates and clinical factors as a cause for acute brain failure leading to specific delirium phenotypes and its associated outcomes [Table 1].[17] The most commonly implicated neurotransmitter abnormalities are deficiencies in acetylcholine, melatonin, and excess in dopamine, norepinephrine and/or glutamate and variable alterations in 5-hydroxytryptamine or serotonin, histamine and/or gamma-aminobutyric acid. Areversible hypometabolism in the global cortex and posterior cingulate cortex is associated with inattention during delirium.[18]
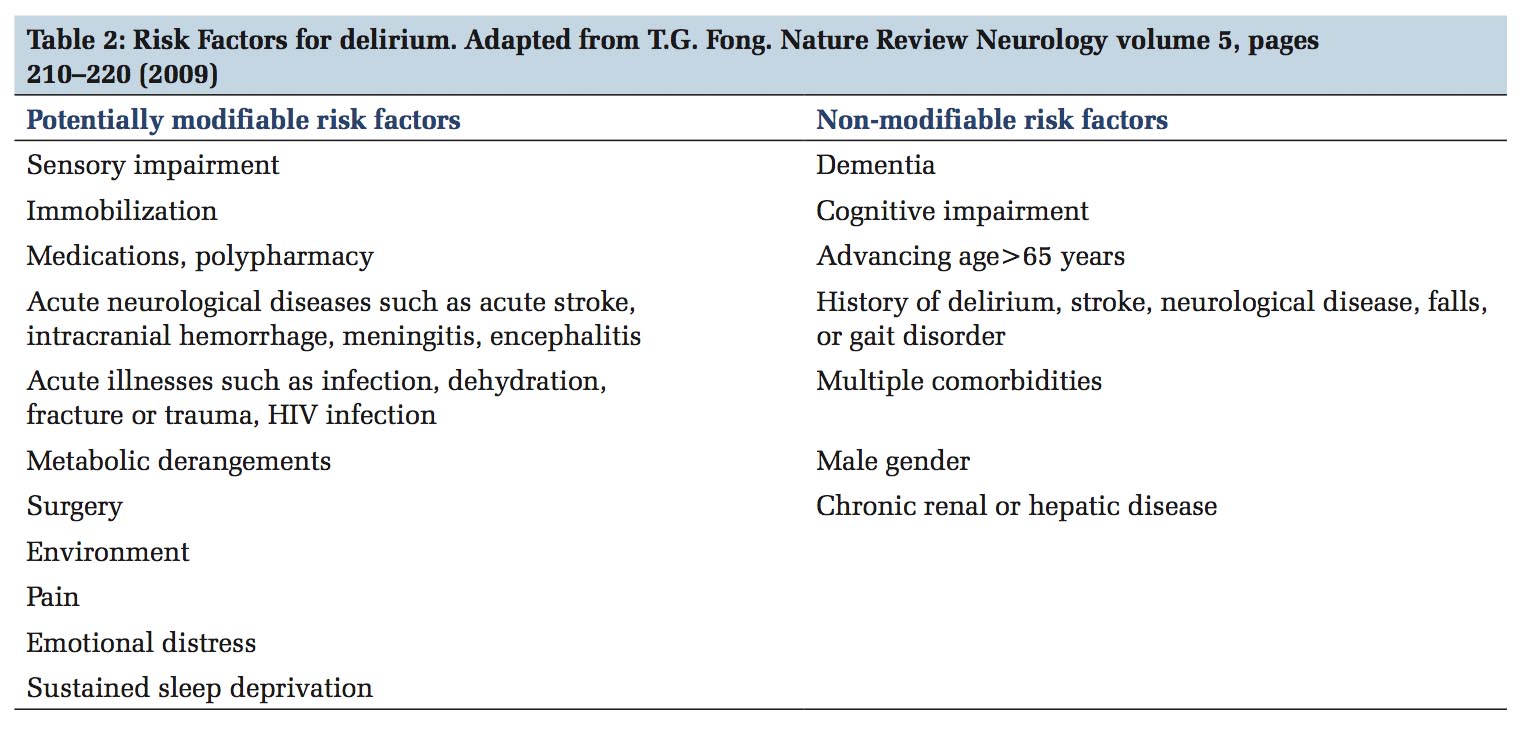
Multiple factors play a role in the development of delirium, and some factors are modifiable [Table 2].[19] Pre-operative factors that predisposes to post- operative delirium are comorbidities, cognitive impairment, fall history, and pre-operative fasting time in patients undergoing joint replacements.[20,21] In vascular surgical patients, those with American Society of Anesthesiologists score >2, renal failure, previous stroke, neurological comorbidity, male gender, older age, lower pre-operative hemoglobin, and longer ICU stays had higher incidences of delirium.[22] Hyperoxic cerebral reperfusion after a period of cerebral hypoxia is also a risk factor in patients undergoing cardiopulmonary bypass during cardiac surgery.[23] Predisposing risk factors for ICU delirium are advanced age, baseline cognitive impairment, increased comorbid disease, frailty, alcohol and drug abuse, and high severity of illness while the precipitating factors include metabolic disturbances, hypotension, sepsis, poor pain control, mechanical ventilation, sleep disturbances, medications such as benzodiazepines, opiates, anticholinergics, steroids, deep versus light sedation, and surgery.[24] A temporal relationship between reduced creatinine clearance and delirium has been demonstrated in the secondary analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study. Acute kidney injury could cause inflammation in remote organ system including the brain as well as reduce clearance of toxic metabolites, medications, and other potential neurotoxins. This study also showed that continuous renal replacement therapy modified the risk of delirium in moderate to severe acute kidney injury.[25]
Based on psychomotor activity delirium has three subtypes:
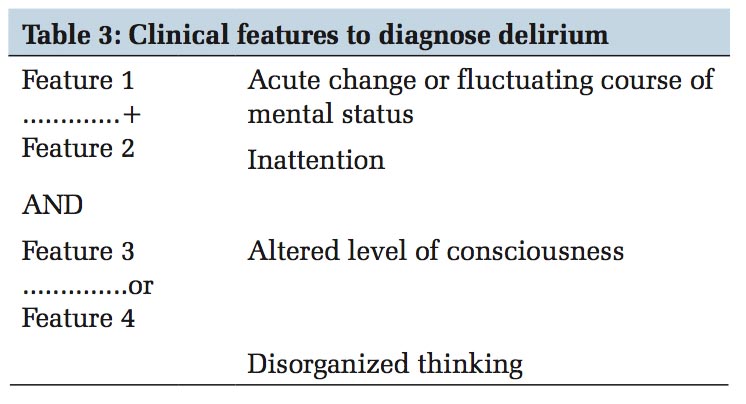
Good history taking from a reliable caregiver to establish baseline mental status, acuity of change and any alteration in consciousness are the foremost steps in recognition of delirium and differentiating from other diagnoses. Next, a careful physical and neurological examination followed by brief cognitive screening tests such as Mini-Cog or the short portable mental status questionnaire and validating with a delirium instrument such as CAM should be performed.[29] Modifications to CAM for adaptation to specific patient populations including medical, surgical, critically ill, emergency department, nursing home, and palliative care have been validated.[30] The CAM was created in 1990byDr.SharonInouye,tobeusedasabedside assessment tool by non-psychiatrists for assessment of delirium. This tool was adapted into CAM-ICU for implementation on ICU patients (both on and offtheventilator).Deliriumisdefinedintermsof four diagnostic features as shown in Table 3. The patient is deemed positive when feature 1 and feature 2 and either feature 3 or 4 are present. The CAM-ICU worksheet and flowsheet are available on http://www.icudelirium.org/. The DRS and its revised version perform best in the psychogeriatric population.[30] Intensive Care Delirium Screening Checklist (ICDSC) comprising eight items checklist based on DSM criteria and features of delirium, was first created and evaluated by Bergeron et al., to be used at bedside by clinicians as a screening tool. The predicted sensitivity and specificity of this tool are excellent at 99% and 64%, respectively.[31] Based on multiple psychometric scoring systems for the delirium assessment tools such as predictive validity, inter-rater reliability, feasibility, and relevance CAM-ICU and ICDSC have emerged as the most sensitive and specific tools for detecting delirium.[32]
Third and most importantly, precipitating and potentially reversible causes for delirium such as dehydration, infection, metabolic derangements, hepatic or renal abnormalities, drug-induced or intoxication or withdrawal related, and Wernicke- Korsakoff syndrome should be carefully reviewed. Further evaluation by neuroimaging, cerebrospinal fluid analysis and or electroencephalogram should be strongly considered if there is a history of falls or suspicion for acute stroke, meningitis, encephalitis, vasculitis or subclinical seizures but they are not needed for confirmation of delirium per se.
Magnetic resonance imaging of the brain in patients with delirium has larger ventricular and sulcal size and higher white matter hyperintensities, suggesting that patients with brain atrophy are at a higher risk of developing delirium.[33] EEG as an objective confirmation for detecting delirium is not very specific and is not routinely indicated. As in Alzheimer’s disease, the EEG during delirium shows increased variability in the spectral domain and decreased complexity.[34] Brain activity and spectral EEG characteristics during delirium varies between two or more states causing increased spectral variability. One or both of these states during delirium may still be more prominent and result in an overall decreased complexity. This reduced complexity is characteristic for neural systems with poor capacity for information processing,[35] and this can be interpreted as a decrease in cognitive capacity. Delirious patients show increased spectral variability, and less complex EEG signals, implicating a decreased cognitive capacity matching the clinical description of delirium as a fluctuating disorder with disturbances in attention and cognition.
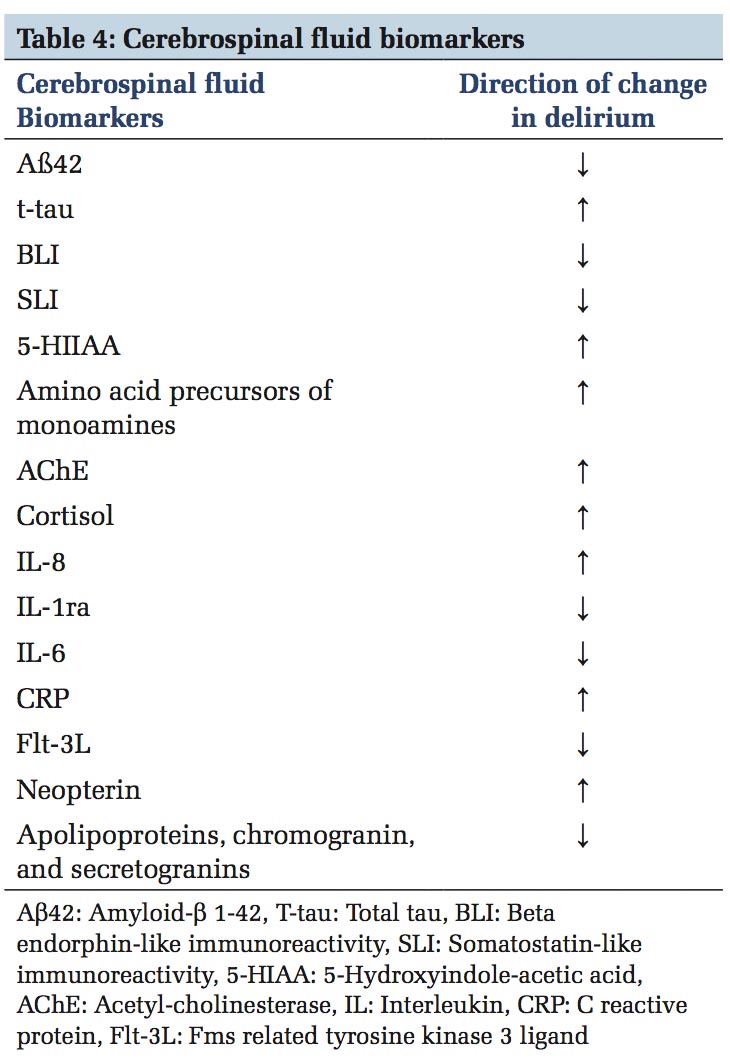
Many biomarkers are being studied; however, there are no current validated markers for clinical application for diagnostic or monitoring purposes. Cholinergic biomarkers are acetylcholine esterase, anticholinergic activity, and butyrylcholinesterase. Inflammatory biomarkers most frequently associated with delirium include interleukin-6, C-reactive protein, interleukin-2, interleukin-1ß, and interleukin-1 receptor antagonist.[29] Some of the CSF biomarkers studied in cases with delirium are briefly summarized in Table 4.[36] Distinction between progressing dementia and depression is important in the elderly as they may coexist and administration of informant questionnaire on the cognitive decline of the elderly, geriatric depression scale may help to discern.[29] Psychosis can mimic delirium, but altered consciousness is not one of its features.
The best prevention strategies for delirium are non- pharmacologic and include providing time, spatial, and situational orientation; family involvement; sensory aids such as glasses and hearing aids; memory clues and cognitive stimulation, adequate pain control, early mobilization, and aiding sleep at night with noise and light reduction. Delirium prevention with haloperidol was recently evaluated in the REDUCE trial (pRophylactic halopEriDol Use for Delirium in iCu patiEnts) which was, a multicenter, placebo-controlled, randomized clinical trial conducted in 21 centers in the Netherlands. Before enrollment, the ICUs had to have best practice strategies to reduce delirium such as early mobilization, improving circadian rhythm, noise reduction, sedation protocols to prevent oversedation, reducing use of benzodiazepines, and the use of hearing and visual aids. Both treatment and placebo groups had delirium in 33% of the patients, and they found no difference in survival rates as well.[37] Similarly, in adult patients undergoing surgery, a single dose of ketamine did not reduce the incidence of post-operative delirium when compared to placebo arm (19.45% vs. 19.82%). There were more post-operative hallucinations and nightmares in the treatment arm, and the effects were additive with increasing doses of ketamine.[38] Opioid-sparing strategies like parecoxib could help in preventing post-operative delirium.[39] A large meta-analysis on the use of statin therapy did not show any beneficial effects in preventing delirium.[40] Medications that potentiates and preserves circadian rhythm like suvorexant, a potent and selective orexin antagonist and ramelteon, a melatonin agonist have been investigated by the DELIRIA-J group in elderly patients admitted for acute care, and both were associated with lower risk of delirium.[41,42] Results from a large multicenter- randomized prophylactic melatonin for delirium in intensive care (Pro-MEDIC) trial which is evaluating the effect of melatonin as a prophylactic agent in the ICU to preserve circadian rhythm and prevent delirium are awaited. Apolysomnography arm will be an insightful explanatory component of the study.[43]
The ABCDEF bundle is a group of interventions developed by the Society of Critical Care Medicine that when applied collectively can help reduce delirium, improve pain management, and reduce long- term consequences for adult ICU patients. Table 5 provides a brief overview, and further information is available at their website, www.iculiberation.org.
A multinational survey from 47 countries on utilization of this bundle, however, has shown that delirium monitoring was implemented in only 70% of the ICUs while only 42% used a validated screening tool. Data from the survey conducted by the Indian Society of Critical Care Medicine found that only 35% of the intensivists reported assessing for delirium and CAM-ICU tool was the most frequently used (22%). In this same survey, nearly all respondents reported using midazolam for sedation (95%) followed by propofol (68%) and dexmedetomidine (60%).[44] ICU quality improvement initiatives to improve communication, collaboration and workflow, education on evidence- based practices, and ICU checklists are some of the interventions which could help delirium prevention and management and improve survival.
Pain control, sedation, and delirium are interconnected while managing critically ill patients. In ICU patients on a mechanical ventilator, the choice and depth of sedation are crucial in prevention and treatment of delirium. Benzodiazepines may cause brain dysfunction through activation of γ-aminobutyric acid central nervous system receptors and potentiate deliriogenic neurotransmitters such as dopamine, serotonin, acetylcholine, norepinephrine, and glutamate. Dexmedetomidine which is a highly selective α2-adrenergic receptor agonist acts at the locus ceruleus and spinal cord level to produce both sedation and analgesia. Dexmedetomidine demonstrated greater delirium or coma-free days than benzodiazepine in the MENDS trial.[45] In this study of 106 critically ill patients by Pandharipande et al., which compared sedation with dexmedetomidine and lorazepam, they found that the patients receiving dexmedetomidine had more delirium/coma-free days than those receiving lorazepam (7 vs. 3; P = 0.01) and lower incidence of coma (63 vs. 92%; P < 0.001). A multicenter trial comparing dexmedetomidine to midazolam by Riker et al. found similar results with incidences of delirium of 54% in dexmedetomidine group and 76.6% in midazolam group (P ≤ 0.001).[46] The PROpofol versus DEXmedetomidine (PRODEX) and Midazolam versus DEXmedetomidine (MIDEX) trials compared sedation using propofol and midazolam head to head with dexmedetomidine and found that dexmedetomidine reduced the duration of mechanical ventilation compared with midazolam and was associated with fewer neurocognitive disorders, improved arousal, cooperation and communication compared to the other two sedatives.[47] A Cochrane review of 7 studies covering 1624 participants comparing alpha-2 agonists (clonidine and dexmedetomidine) versus traditional sedatives in mechanically ventilated patients, found reduced duration of mechanical ventilation and ICU length of stay. There was no evidence for a beneficial effect on risk of delirium or mortality rates, but the heterogeneity was high in these studies. Bradycardia was found to be the most common adverse event.[48] A more recent meta- analysis showed potential benefits in reducing the duration of mechanical ventilation and lowering the risk of delirium.[49] Dexmedetomidine to lessen ICU agitation (DahLIA) study was a randomized, double- blind placebo-controlled trial by the ANZICS group showed statistically significant, accelerated resolution of delirium (median 23.3 h vs. 40.0 h).[50] In non-intubated patients with hyperactive delirium and delirium refractory to other medications like haloperidol, dexmedetomidine can be used, and even though the direct cost of the medication is high, it is cost-effective when its impact on lowering the ICU length of stay is considered.[51]
The current trend is the use of analgesia-based sedation regimens which is associated with an increase in days without mechanical ventilation; however, the occurrences of delirium may be more frequent.[52,53]
For treatment of acute delirium, antipsychotics such as quetiapine, haloperidol, olanzapine, and ziprasidone may help in reducing the duration of delirium. However, they come with significant side effects especially in the elderly, the most dangerous being prolonged QTc interval and increased risk for arrhythmias. Ramelteon, a melatonin receptor agonist, may help reduce the as-needed antipsychotic use for agitation.[54]
Clonidine, an alpha-2 partial agonist stimulates the presynaptic alpha-2 adrenoreceptors within the brain stem, decreasing the norepinephrine release while enhancing parasympathetic activity.[55] Utility of clonidine as an agent to reduce been extrapolated from studies of dexmedetomidine which showed reduced incidence of agitation and delirium when compared with lorazepam and midazolam.[45,46] agitation has
The Society of Critical Care Medicine has complied PAD guidelines for critically ill adult patients.[32] The quality of evidence for each statement and recommendation was ranked as high (A), moderate (B), or low/very low (C). The strength of recommendations was ranked as strong (1) or weak (2), and either in favor of (+) or against (–) an intervention. In relation to delirium, some of the important statements are summarized in Table 6.
Delirium outcomes range from mortality, a variable degree of cognitive and functional deficits to full functional recovery. In addition, it is associated with prolonged length of stay, prolonged periods on mechanical ventilation,[7] high rates of unplanned and unintentional device removal, falls, incontinence, significant emotional distress, and long-term consequences such as impaired physical functioning, disability in activities of daily living,[56] need for long- term care placement, caregiver burden, decreased quality of life, post-traumatic stress disorder, cognitive decline, and increased risk of dementia; all of these leading to escalating public health-care costs. Prolonged duration of delirium is a risk factor for long-term cognitive impairment. BRAIN-ICU study which enrolled 821 patients found an association of duration of delirium to global cognitive and executive functioning score 3 and 12 months after an episode of critical illness.[57] A neuroanatomical basis was demonstrated by VISIONS investigators using brain magnetic resonance imaging. Patients with longer duration of delirium had greater brain atrophy as evidenced but a larger ventricle to brain ratio both at hospital discharge and at 3 months follow-up. Poor executive functioning and visual attention at 12 months post-delirium were predictable by low volumes of superior frontal lobes, thalamus, and cerebellum.[58]
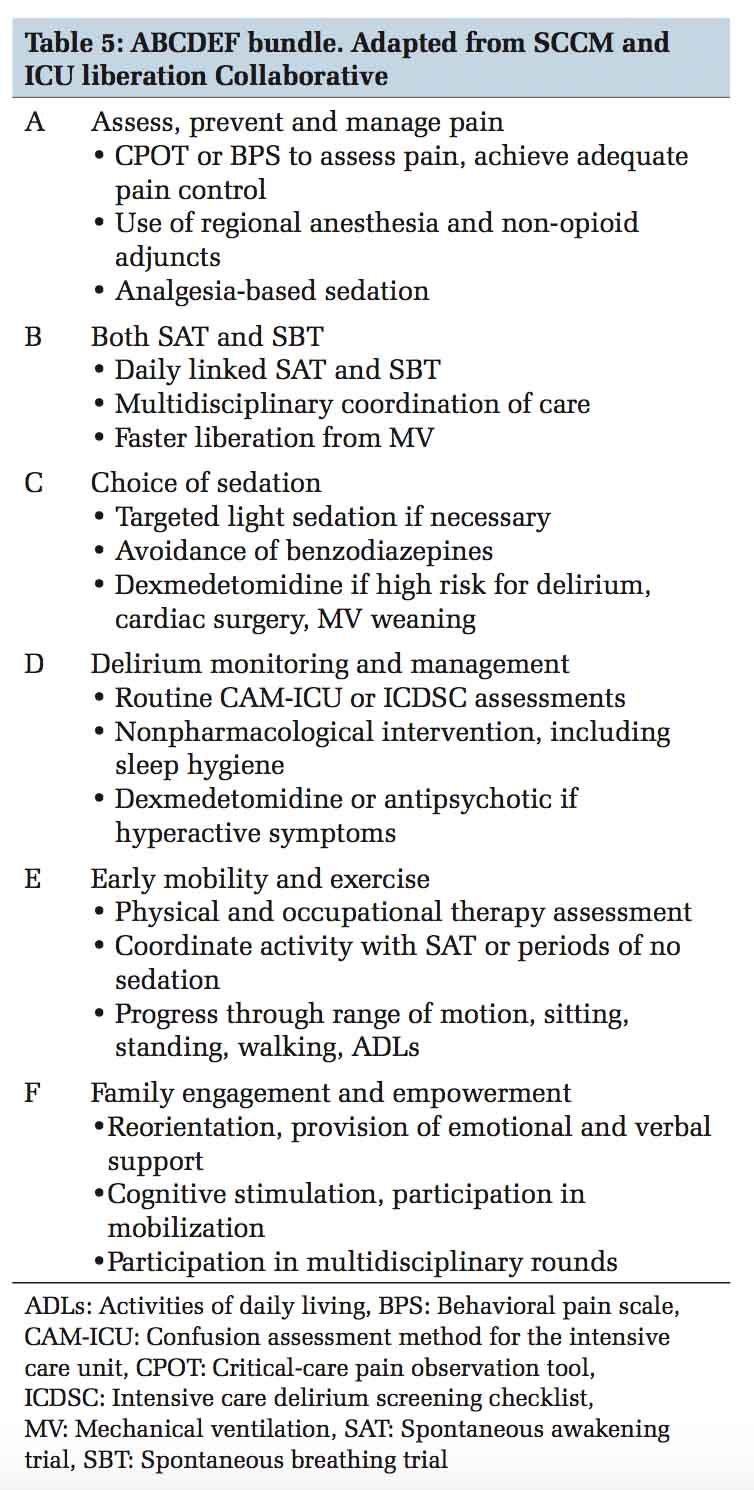
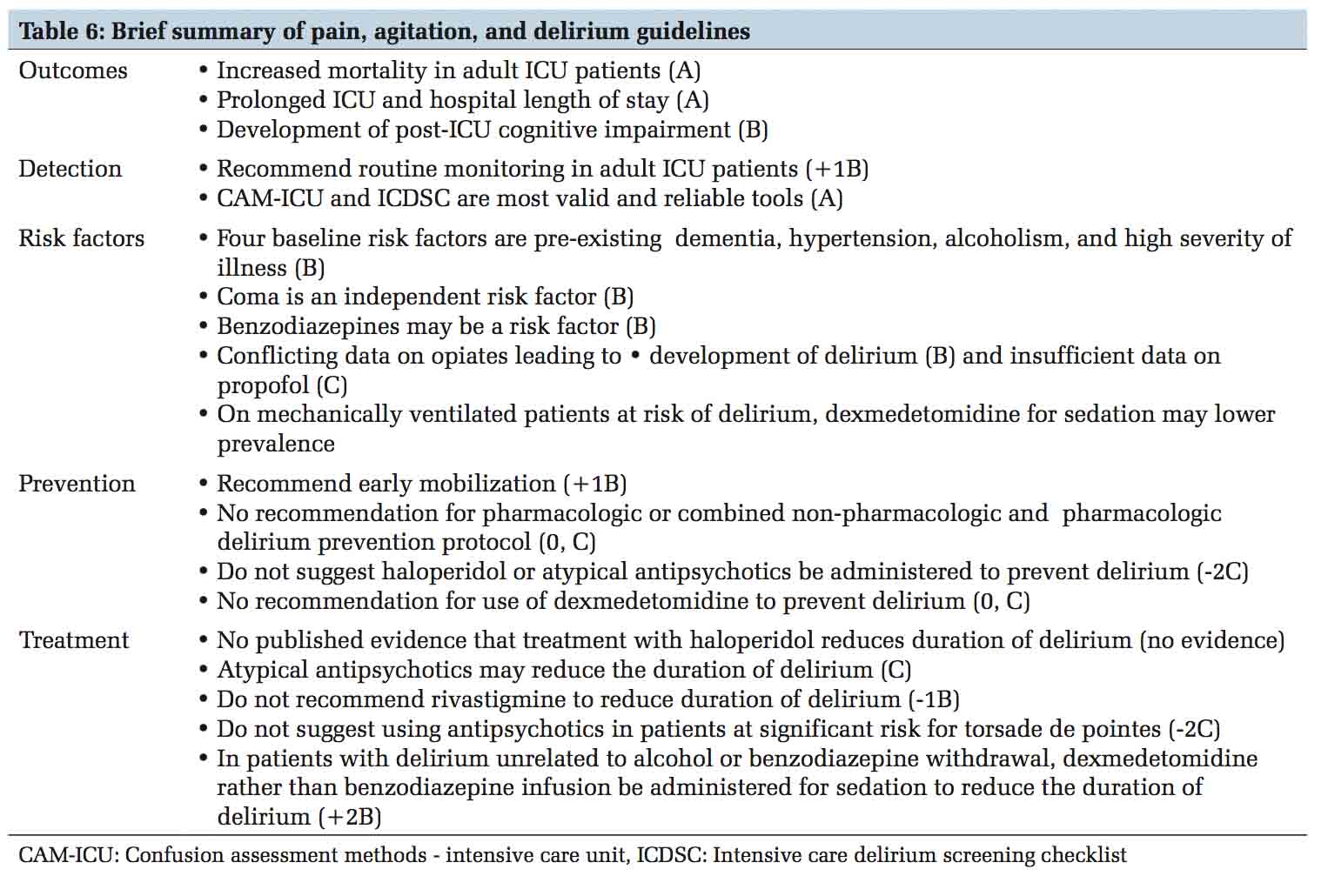
Approach to delirium requires a planned and protocolized approach in the ICU. Provider education, improving team communication, standardizing care processes, prioritizing periodic delirium monitoring, lightening sedation and providing daily sedation interruption, pain control, facilitating early mobilization and using non- pharmacologic preventive strategies are some of the quality improvement techniques that ICUs should strive to achieve to manage ICU delirium.
Janice L. Zimmerman MD, MACP, MCCM Professor of Clinical Medicine Weill Cornell Medical College, Head, Critical care division, Department of Medicine Houston Methodist Hospital.
Subscribe now for latest articles and news.