

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2015.v01i02.002
Year: 2015, Volume: 1, Issue: 2, Pages: 9-13
Original Article
Vidya V Bhat1, Neha P Chandel2, B S Bhat2, M D Geetha3
1Department of Obstetrics and Gynecology, Radhakrishna Hospital & IVF Centre, Bengaluru, Karnataka, India,
2Consultant, Radhakrishna Hospital & IVF Centre, Bengaluru, Karnataka, India, 3Department of Embryology, Radha Krishna Hospital & IVF Centre, Bengaluru, Karnataka, India
Address for correspondence:
Neha P Chandel, ICOG-Gynae Endoscopy Fellow, Radhakrishna Hospital & IVF Centre, Bengaluru - 560 085, Karnataka, India.
E-mail: [email protected]
Objective: To investigate the role of trans- abdominal ultrasound (TAS) guidance in intrauterine insemination (IUI).
Materials and Methods: A multicentre retrospective study was conducted at three centres in Bangalore, Karnataka from November 2012 and December 2014. 146 couples with unexplained infertility and mild male factor infertility were randomized using a computer-generated random numeric table into two groups: Group 1 who underwent TAS-guided IUI (n =73) and Group 2 who underwent classical blind IUI (n =73). 480 IUI cycles were performed for both the groups in total.
Results: Of the 480 IUI cycles, 228 were carried out as TAS-guided, while 252 cycles were performed as the classical blind procedure. The difference in pregnancy rates of Group 1 and Group 2 was statistically significant (22.9 and 10.6% respectively (P = 0.00036), thereby indicating that TAS-guidance significantly improves pregnancy rates in IUI. In Group 1, 10.8% cases were difficult whereas in Group 2, 31.2% cases were difficult (P = 0.00001).
Conclusion:Artificial insemination, intrauterine insemination, ultrasound.
KEY WORDS:Artificial insemination, intrauterine insemination, ultrasound.
IntroductionIntrauterine insemination (IUI) is a therapeutic procedure for unexplained infertility and infertility caused by mild to moderate female and male pathologies, with typical per cycle pregnancy rates (PRs) of 10-20%. Various published studies on IUI discuss about ovarian stimulation and sperm management, but less attention has been given to insemination technique. In treatment of infertility, during embryo transfers, trans-abdominal ultrasound facilitates atraumatic embryo placement[1] and higher PRs have been reported with the use of abdominal ultrasound during the transfers as compared to transfers based on clinical methods only.[2-4] Ultrasound facilitates visualization of the cervicouterine angle and thus reduces the number of difficult cervical catheterizations,[4] as well as manipulations. Furthermore, ultrasound guidance prevents the catheter from impacting the uterine fundus by allowing visualization of the endometrial cavity. Cervical as well as endometrial manipulations increase uterine contractions due to the secretion of prostaglandins and or oxytocin.[5-7] Expulsion of >40% of the volume introduced into the uterine cavity in IUI has been reported due to these contractions, thus reducing the number of spermatozoa with access to the tubes and therefore the success of the procedure.[8,9] An important variable that influences the outcome of IUI is the number of inseminated spermatozoa.[10,11] Incidence of infertility is on a rise due increased representation of females in the workplace, delay in marriages, stress and ignorance. In India, unlike the olden days, due to increased efforts of the government and NGO’s to create awareness, lot of couples report and seek consultation for infertility issues. However, due to lack of published literature from the subcontinent, magnitude of the problem and the result of these procedures in treating infertility is unknown. To our knowledge, not many studies, especially from the subcontinent have analyzed and compared the role of ultrasound guidance in IUI. Thus, we initiated this study to analyze the role of abdominal ultrasound during IUI and to compare the PRs of ultrasound-guided with non-ultrasound-guided inseminations. Materials and Methods We initiated this study in 2012 following clearance from the Radhakrishna Hospital Ethical Committee and obtaining informed consent from the patients. We performed IUI in 146 couples at our Infertility Center from November 2012 to January 2014, with a total of 480 IUI cycles performed. Inclusion criteria Women < 40 years, normal uterine cavity, at least one patent tube, basal follicle stimulating hormone (FSH) < 10 mU/ml, mild female factor (polycystic ovary syndrome), unexplained infertility, male factor infertility: Oligospermia - at least 5 million/ml motile spermatozoa recovered after sperm preparation for IUI with Husband’s semen (IUI-H), post semen preparation count < 5 million/ml for IUI with donor semen (IUI-D), mild teratospermia, mild asthenospermia. Exclusion criteria Women >40 years, previous 3 or more IUI failures, previous in vitro fertilization (IVF) failures, severe female factors like Stage 3, 4 endometriosis, hydrosalpinx uni/bilateral, premature ovarian ageing, patients not willing for IUI, patients with severe male factor infertility and not willing for donor semen. Methodology Randomization protocol Following institutional ethical committee clearance and obtaining Informed consent from the patients, patients were counseled and motivated for the IUI procedures. 146 patients comprised the study population. They were randomized into two groups using a computergenerated random numbers. In Group 1 (n = 73) patients underwent ultrasound-guided IUI and in Group 2 (n = 73) patients underwent classical blind IUI. The infertility work-up, i.e. transvaginal ultrasound, basal hormone tests (on 2nd day of the cycle), hysterosalpingography and sperm analysis were done for all the couples. The average duration of infertility of all the couples was 3.3 ± 2 years. IUI protocol IUI was done with husband’s sperm (IUI-H) in cases where after the sperm preparation, the post wash counts were at least 5 million motile spermatozoa or with donor sperms (IUI-D) in cases of azoospermia, post wash counts < 5 million motile spermatozoa, failure to recover spermatozoa during testicular biopsy. The stimulation protocol was the same for all patients, consisting of a daily subcutaneous injection of 225 IU of purified FSH, started on day 2 of the menstrual cycle.[12] The ovarian response to stimulation was regulated by adjusting the dose according to transvaginal folliculometry and plasma estradiol assay. When at least one follicle had a diameter of 16-20 mm, we administered one subcutaneous dose of 0.25 mg/day of cetrorelix, maintaining this dose until the day on which human chorionic gonadotropin (HCG) was administered.[13] We administered 250 μg of recombinant HCG (rHCG) when there were at least two follicles with a diameter of ≥17 mm, with estradiol level ≥500 pg/ml (≥2200 pmol/l). The treatment cycle was cancelled if there were <2 or >5 follicles. Two inseminations per cycle were carried out, firstly at 37-39 h after rHCG injection and secondly at 48-50 h after HCG injection,[14] with a maximum of six insemination cycles per patient. All the semen samples were prepared with density gradient method. In both the groups, the patients attended the appointment with a full bladder, and the same catheter model was used for the procedure in both instances: The Frydman soft model, which has two channels: Rigid outer (with a cap) and flexible inner; all IUIs (both ultrasound guided IUI and clinical blind IUI) were performed by staff gynecologists and the majority (90%) were carried out by the same gynecologist. In patients undergoing ultrasound-guided insemination, the external (rigid) sheath of the catheter was molded in advance according to the angle, and introduced into the cervical cavity to 1 cm past the internal cervical orifice. Via this sheath, under abdominal ultrasound guidance, we introduced the flexible inner catheter into the uterine cavity, until the tip was located at a distance of 1 cm from the fundus. Abdominal ultrasound was performed by means of general electric medical systems logic 3 ultrasound machine with the 3.5C abdominal probe. In Group 2, cervical catheterization was performed, until the resistance of the internal cervical orifice was passed, introducing the internal catheter according to clinical criteria, based on a hysterometry carried out during the infertility work-up. In no cases, was cervical tenaculum or hysterometer needed at the time of the procedure (either in ultrasound IUI or in classical IUI). Pregnancy was defined by visualization of the gestational sac at vaginal ultrasound 3-4 weeks after insemination. The luteal phase was supplemented with vaginal micronized progesterone at doses of 200 mg every 12 h. Observations Among 146 patients, 118 patients (80.82%) underwent IUI with husband’s sperm (IUI-H) whereas donor sperms (IUI-D) were used in 28 patients (19.18%). None of the couples underwent any previous infertility treatments. In the Group 1 (ultrasound-guided group), 228 cycles were carried out and the in Group 2 (classical blind) group, 252 cycles were carried out. The patient characteristics of the both the groups were similar in terms of age, hormonal status, ovarian stimulation, and sperm parameters (Table 1). The number of women completing six cycles of treatment was similar in both groups: 14 in Group 1 (14 with husband’s sperm and zero donor) and 15 in Group 2 (13 with husband’s sperm and two donors).The PRs per cycle was 22.9% (52/228) in the ultrasound-guided group and 10.6% (27/252) in the classical IUI group (Table 2); 95% confidence interval for the difference in PRs was 5-9% (0.05- 0.09) P = 0.00036. There were significant differences in PR per woman, the first‑cycle PR as well as in the cumulative PR in both the populations. There were significant differences in the PR between ultrasound and non-ultrasound groups when analyzed according to the indication for IUI. Abortion rate was higher in Group 2, as well as multiple PR. High-order multiple pregnancy was also higher (one triplet in the non-ultrasound guided group versus no triplets in the ultrasound-guided group). 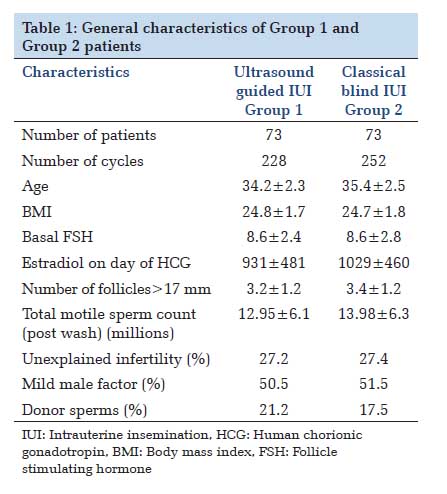 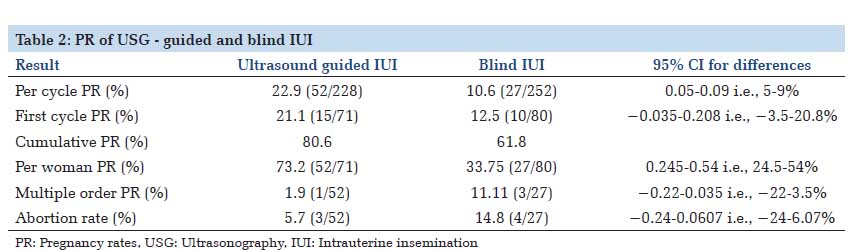 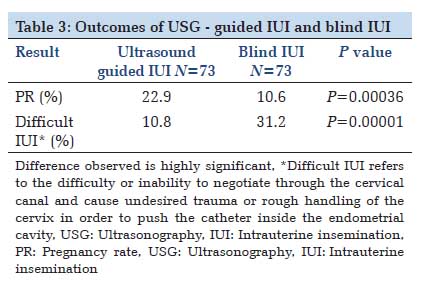 |
DiscussionIn infertility management, IUI is a technique similar to embryo transfer in IVF, which requires cervical catheterization to access the uterus. In recent years, although a number of studies have been associated with embryo transfer prognosis, the effect of IUI insemination technique has received little attention. Theoretically, IUI performed under ultrasound guidance could be associated with increased PRs, since this may reduce cervical and endometrial damage as well as bleeding, thus reducing the release of prostaglandins as well as uterine contractions. The use of ultrasonography as a support for assisted reproduction techniques has been widely used in IVF. Difficulty of the transfer, presence of blood in the catheter and uterine contractions all reduce the implantation rates.[15] The use of abdominal ultrasound during transfer provides direct visualization of the movement of the catheter inside the endometrial cavity, reducing the frequency of difficult transfers and minimizing endometrial damage. However, its use in IUI has not been studied much. Both IUI and embryo transfer require cervical catheterization and deposition of the end product of the reproductive technique inside the endometrial cavity. IUI results are influenced by a number of parameters: Female and male conditions, sperm characteristics and preparation, infertility status, ovarian stimulation and methodology, as has been demonstrated in a number of studies.[14,16 In our country, technique of IUI, however, has received little attention. In classical blind IUI, the processed specimen is deposited using a blind technique, dependent on the skill of the physician. In IVF, it has been shown that blind transfer leads to inadvertent contact of the tip of the catheter with the uterine fundus in 17.4% of cases,[17] provoking an increase in uterine contractions;[6] expulsion of >40% of the volume introduced into the uterine cavity has been reported.[18] Furthermore, the presence of contractions may eliminate almost half of the spermatozoa, unbeknown to the clinician, and thereby limiting the results.[11] Another possible cause of uterine contractions is the handling of the cervix in achieving catheterization. The ultrasound visualization of cervico-uterine angle facilitates and allows the catheter to be adapted to the angle, thus avoiding excessive manipulations and resulting in a traumatic catheterization. Out of the prognostic factors for IUI thus, a full bladder, the number of motile spermatozoa deposited and the site of deposition of the sample in the uterus are important. Full bladder exerts an insemination facilitating effect as it passively straightens the cervical canal.[19] The processes that follow the release of embryo/ spermatozoa into the uterine cavity differ between IVF and IUI. In IVF, it has been reported that it is important to deposit the embryos in a specific place in the uterine cavity,[20] which is in many cases close to the site of implantation. However, in IUI, spermatozoa must still ascend through the fallopian tube, as far as the ampullary portion, where fertilization occurs. In IVF, cervical and endometrial damage could interfere with implantation, either directly or via prostaglandin release. However, in IUI, since implantation occurs approximately 7 days after insemination, cervical and endometrial damage are likely to have been repaired by this time. Rapid transfer also increases the chance of pregnancy in IVF.[21] The object of our study was therefore to ascertain whether using ultrasound guidance during IUI could increase the PRs. The control and study populations proved to be comparable regarding the main demographic and clinical parameters. Our study found statistically significant differences in PRs with ultrasound-guided IUI and classical blind IUI. The PRs per cycle were higher significantly in ultrasound guided IUI group (22.9 vs. 10.6%).With regard to PR per woman also, significant differences in ultrasound guided IUI and blind IUI (73.2 and 33.75%) were observed (Table 3). In our study, the use of ultrasonography during IUI facilitated the technique. Though ultrasonography requires investment, like an ultrasound probe and specialized personnel (ultrasound technician), but results with this technique are promising. Ultrasound is a portable tool with no radiation exposure and which is moderately expensive. We consider atraumatic catheterization necessary for preventing uterine contractions, but this can be achieved both by requesting a moderately full bladder in order to reduce the cervico-uterine angle and by molding the catheter to an angle of 30-60°, which corresponds to the angle observed in majority of the patients. |
ConclusionOur results suggest that in the treatment of infertility, ultrasound guided IUI enhances the PRs as compared to blind insemination. Thus, we recommend the systematic use of trans-abdominal ultrasound guidance during IUI, especially in the Indian scenario due to financial and psychological concerns of the patients to get successful results. |
Subscribe now for latest articles and news.