

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2019.v05i01.005
Year: 2019, Volume: 5, Issue: 1, Pages: 21-26
Original Article
Emine Nedime Korucu1, Dudu Erkoc-Kaya2, Esma Menevse3, Hilal Arikoglu2
1Assistant Professor, Department of Molecular Biology and Genetics, Faculty of Science, Necmettin Erbakan University, Konya, Turkey,
2Department of Medical Biology, Faculty of Medicine, Selcuk University, Konya, Turkey,
3Department of Medical Biochemistry, Faculty of Medicine, Selcuk University, Konya, Turkey
Address for correspondence:
Dr. Emine Nedime Korucu, Assistant Proffesor, Department of Molecular Biology and Genetics, Faculty of Science, Necmettin Erbakan University, Konya, Turkey. Phone: +905055610760. E-mail: [email protected]
Background: Naphthoquinones have protective effects through different mechanism against to human malignancies including pancreatic cancer. One of these mechanisms is to avoid reactive oxygen species (ROS) production. Changes in enzymatic (superoxide dismutases, catalase [CAT], ascorbate peroxidase, glutathione peroxidase, and glutathione reductase) antioxidant systems, such as formation of an oxidative biomarker (H2O2, malondialdehyde, ischemic modified albümin, etc.) have a critical role in ROS mechanism. According to this knowledge, we evaluated the anticancer and antioxidant activity of the juglone.
Objectives: The aim of this study was to investigate the cytotoxic activity of juglone and to investigate its effect on antioxidant activity in BxPC-3 and PANC-1 pancreatic cancer cell lines.
Materials and Methods: The cytotoxic activities of juglone on cell viability were investigated on pancreatic cancer cells using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability test. One of the antioxidant enzymes, CAT activities and antioxidant status marker reduced glutathione (GSH) levels were measured by spectrophotometric analysis. We compared the groups as juglone treatment and control groups (no treatment) at different hours (24th, 48th and 72th h).
Results:Juglone reduced the cell viability of human pancreatic cancer cells in a concentration-dependent manner. Juglone supplementation showed an antiproliferative effect toward pancreatic cancer cell lines at IC50 values. The IC50 of juglone on BxPC-3 and PANC-1 pancreatic cell line was 21.05 μM and 21.25 μM, respectively. Furthermore, juglone had a significantly higher degree of enzymatic activity to compensate the oxidative stress. CAT activity was found a significant increase compared to the control group at 24, 48, and 72 h in PANC-1 cells; it was found a significant increase at 72 h in BxPC-3 cell line. Reduced glutathione level is decreased at 24 and 48 h while at 72 h GSH level is increased in BxPC-3 cell line after juglone treatment compared to the control group. In PANC-1 cell line, GSH level is increased at 24 and 48 h, but it was decreased at 72 h compared to the control group.
Conclusions: Our results indicate that juglone can be a potent anticancer molecule and may prove essential in pancreatic cancer therapy. Juglone may play a central role in antioxidant system defense in pancreatic cells.
KEY WORDS:Catalase, juglone, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, reduced glutathione, pancreatic cancer.
Pancreatic cancer is known to spread rapidly and rarely detected in early stages. Until the last phases, no signs or symptoms are observed, and in the advanced stage, it is impossible to remove the tumor. This type of cancer is known to be aggressive and migrate to different sections of the body quickly. If metastasis of pancreatic cancer could be stopped, it would be possible to manage the tumor without searching for additional tumor spread, and if this treatment could be combined with drug resistance decrease, it could be used as a good treatment option for pancreas cancer.[1] In addition, surgery at pancreatic cancer may be not possible and long-time survival after surgical operation is low.[2,3] Since the survival of patients with pancreatic cancer is low, people need to find new therapeutic agents.
Naphthoquinones, which are called secondary metabolites, are available in nature. They obtained through bacteria, fungi, and plants. They have several biological activities to contribute oxidative stress through redox cycling or to interact with cellular macromolecules and biological process.[4-6] Juglone is a natural product with the chemical structure of 5-hydroxy-1, 4-naphthoqulnone, and it is extracted from leaves, roots, nut-hulls, wood, and bark of Juglandaceae family.[7] Earlier studies showed that Juglone demonstrates allelopathic activities besides its antiviral, antibacterial, and antifungal activities. Its anticancer effects have been shown in several studies on human tumor cell lines, which includes pancreatic, cervical, leukemia, gastric, melanoma, prostate, glioblastoma, and lung cancer cell line.[8-13]
Oxidative stress causes the production of reactive oxygen species (ROS) in the enzymatic and nonenzymatic pathway. In the enzymatic pathway, they produce some dangerous products as superoxide anion, hydrogen peroxide, and hydroxyl radical. ROS can involve oxidative damage to lipids, proteins, DNA, and RNA, finally leading to cell death by apoptosis or necrosis.[14] Cancer cells are more vulnerable induced by exogenous ROS-producing agents. To decrease the ROS level, cells have abilities against to ROS defense system through superoxide dismutases (SODs), catalases (CATs), and peroxidases (POXs). These molecules are important signaling messengers playing key roles in controlling a broad range of physiological processes, such as cellular growth and development, as well as adaptation to environmental changes.[15]
In the cell, primary enzymatic defense components consisting of CAT, ascorbate peroxidase (APX), and glutathione reductase (GR) protect cellular structures by scavenging extreme ROS levels, glutathione peroxidase (GSH-Px) play an important role in protecting cell membranes from oxidative damage.
In this study, it is aimed to show the cytotoxic activity of juglone in the BxPC-3 and PANC-1 pancreatic cancer cell lines and also to investigate its effect on antioxidant activity through evaluating enzyme activity of CAT and reduced form of GSH. We showed the ability of juglone how it has contributed to the formation of ROS and suggested that these molecules play an important role in the proliferation of pancreatic cancer cell lines.
Cell culture
BxPC-3 and PANC-1, human pancreatic cancer cell lines, obtained from the ATCC (Manassas,VA, USA), were cultured in RPMI-1640 and DMEM medium, containing 10% fetal bovine serum and 1% penicillin/streptomycin, respectively. They were cultured at 37°C in a humidified atmosphere of 5% CO2-95% air. Juglone was purchased from Merck.
Cell viability assay
Antiproliferative effect of juglone on pancreatic cancer cells was exerted by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability test. Juglone stock solution (20 mM) was prepared in DMSO (final concentration ≤0.1%) and then diluted with the culture medium to get the desired concentrations. The cells (5000/well) were seeded into 96-well plates and treated with various concentrations of juglone (0, 5, 10, 15, 20, 30, 40, and 50 μM) for 24 h. At the end of the incubation, 10 μl MTT solution (5 mg/ml) was added to each well. After the incubation at 37°C for 4 h, the resulting formazan crystals were dissolved with DMSO. The absorbance was read at 570 nm in an ELISA reader. The concentration of juglone that inhibits 50% cell viability (IC50) was determined. IC50 values were used in the present study.
Enzyme activity assay
BX-PC3 and PANC-1 cells were seeded into 25 mm flasks as 106 cells per flasks and treated with 21.05 and 21.25 μM doses, respectively, and triplicate samples were prepared for each analyze. Cells were incubated for attaching 24 h. After 24 h incubation, cells were treated with juglone at following 24, 48, and 72 h. Then, media were removed, and cells were washed with PBS. Cells were dissolved in RIPA buffer and they were prepared for analyze through centrifugation (14,000 × rpm) at 4°C for 30 min. Supernatants were used for the determination of protein content and enzyme activities. Total CAT (EC 1.11.1.6) activity was estimated according to the method of Bergmeyer. The unit of CAT was given as U/mg protein. GSH levels were analyzed according to the method of Ellman.[16] GSH levels were expressed as mg GSH/mg protein. The total soluble protein content of the enzyme extracts was determined[17] using bovine serum albumin as a standard. All spectrophotometric analyses were conducted on a Shimadzu spectrophotometer (UV 1800).
Statistical analysis
Each experiment has been done in triplicate. Data were analyzed independent test using Kruskal–Wallis test. All values were expressed as means ± standard deviation. P < 0.05 was considered significant.
Effect of juglone on cell viability
The antiproliferative effect of juglone was determined using the MTT cell viability test. Incubation with juglone for 24 h significantly decreased cell viability when compared to control. Juglone inhibited the cell viability of pancreatic cancer cells in a dose- dependent manner as shown in Figure 1. In BxPC-3 cell line, IC50 dose of juglone was determined to be 21.05 μM and in PANC-1 cell line, it is 21.25 μM for 24 h. We administered in CAT activity and GSH levels at these IC50 doses.
Effect of juglone on antioxidant activity
CAT activity shows changing at widely across cell lines. CAT activity is known to exhibit lower activity in tumor cells, where CAT expression ranges on the order of 10-100 fold times more for normal cells in comparing to some tumor cells. For example, prostatic and pancreatic cancer cells have lower levels of superoxide dismutase (SOD) and CAT than normal tissue.[18-20]
According to the CAT enzyme activity, treatment of juglone for 24 h, 48 h, and 72 h increased the CAT levels of pancreatic cancer cells in a time-dependent manner as shown in Figure 2. This study showed that a significant increase in CAT activity was found at 24, 48, and 72 h in PANC-1 cells. The CAT capacity of the cells was increased by 1.96, 2.62, and 7.63, respectively, compared to the control (P < 0.05).
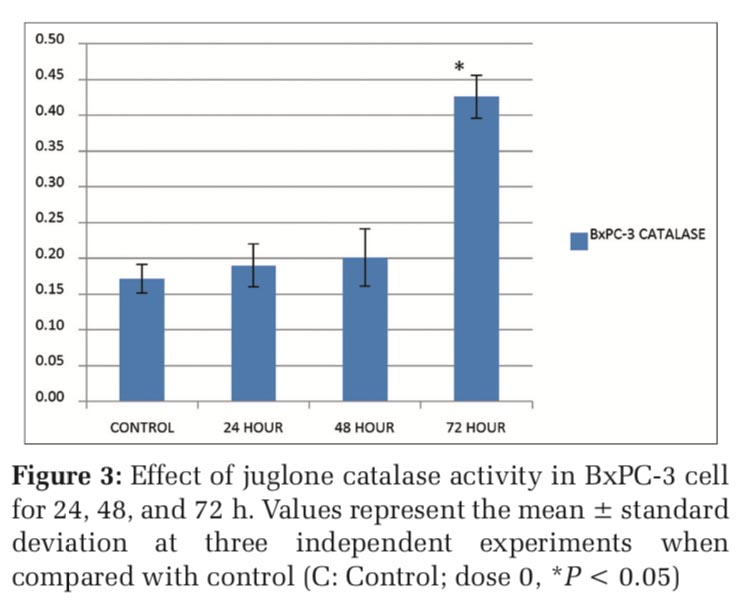
As shown in Figure 3, after the treatment of 21,05 μM juglone, the catalase values were 0.17, 0.19, 0.20 and 0.43 U/mg protein respectively in control, 24th, 48th and 72th hours in BxPC-3 cells. The catalase activities of BxPC-3 cells were increased as 2,53 fold times at 72 hour compared to the control. According to these findings, we concluded that juglone treatment increases antioxidant activity especially at 72th hour in BxPC-3 cells.
The effect of juglone on activity of reduced GSH was determined using spectrofotometric methods. The GSH values were 20.56, 31.59, 28.05 and 15.48 mg GSH /mg protein respectively in control, 24th, 48th and 72th hours in PANC-1 cells. A significant increase in the GSH levels of PANC-1 cell line was observed after juglone treatments as 1.54 and 1.36 folds (p < 0.05) at 24 and 48 hour respectively while we determined a significant 0.75 fold decrease for GSH level at 72 hour compared to the control group as shown in Figure 4.
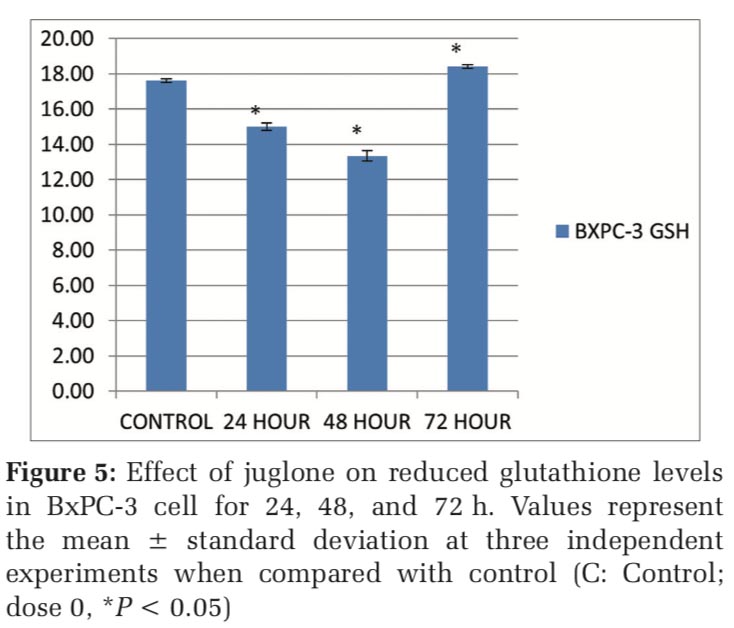
The GSH values were 17.62, 15, 13.34 and 18.41 mg GSH /mg protein respectively in control, 24th, 48th and 72th hours in BxPC-3 cells. According to the results in BxPC-3 cells, as shown in Figure 5, reduced glutathione (GSH) level was decreased as 0.85 and 0.76 folds respectively at 24 and 48 hour while at 72 hour GSH level was increased as 1.04 folds in BxPC-3 cell line after juglone treatment compared to the control.
The ”antioxidant defense systems” identified as the prevention system from formation of reactive oxygen species which are harmful molecules, plays important role for detoxification and prevents damages in cellular systems. Intracellular glutathione which is one of the non-enzymatic antioxidant systems plays an important roles. Glutathione peroxidase (GPx), which is not dependent on selenium, has a mostly duty to metabolize organic hydroperoxides.[21]
During these metabolizing reactions GSH, as to be hydrogen donor, GSH is oxidized whereas H2O2 and hydroperoxides are reduced. Oxidized glutathione (GSSG) in the presence of GR enzyme is reduced back to reduced glutathione (GSH).[22] When cells are exposed to oxidative stress, GSSG levels are increased. The effect of juglone on the activity of reduced GSH was determined using spectrophotometric methods. A significant increase in the GSH levels of PANC-1 cell line was observed after juglone treatments as 1.54 and 1.36 folds (P < 0.05) at 24 and 48 h, respectively. We also found the GSH level at 72 h was 0.75-fold compared with the control group.
According to the results in BxPC-3 cells, GSH level is decreased to 0.85 and 0.76 folds, respectively, at 24 and 48 h while at 72 h GSH level is increased to 1.04 folds in BxPC-3 cell line after juglone treatment compared to the control.
Several studies have shown that natural products are beneficial to compensate against specific human malignancies including pancreatic cancer. Therefore, recent studies have lead to discover anticancer agents from natural sources. Juglone has been affected several signal pathway in Chinese treatment for various tumors, through activating caspase pathway, and the increase of ROS. At now, it is found that juglone could inhibit cell proliferation and induce ROS production in pancreatic cancer cells.
ROS are the known mediators of intracellular signaling cascades. The excessive production of oxidative stress is caused ROS production, such as superoxide anion radical, hydrogen peroxide and the hydroxyl radical as a result of this, cells loss cellular function and head for apoptosis or necrosis.[23] In animals, CAT is an intracellular enzyme located mainly in peroxisomes.[24] In our study, we aimed to determine the effects of juglone on the enzymatic defense of ROS mechanism in human pancreatic cancer cells based on the lack of the studies associated with the antioxidative effect of juglone at time-dependent effect.
Hu et al. l used photodynamic therapy by coloading with CAT and methylene blue (MB), a new type of oxygen self-sufficient PDT platform, a zeolite- CAT-MB nanocapsule (ZCM nanocapsule).[25] They found that the local PC cells are completely killed, and no therapy-induced toxicity and recurrence are observed.
Erudaitius et al. determined intracellular H2O2 concentration for several cell lines. The results demonstrated that CAT concentration and plasma membrane permeability showed significant diversity at cell lines. Intracellular per extracellular H2O2 concentration ratio did not significant across cell lines. Therefore, it may be investigated to determination of intracellular H2O2 concentration through different methods.[26]
In this study, they noticed the effect of juglone treatments on the expression level of Cat1, Cat2, and Cat3 genes, in maize and wheat seeds. It was shown that juglone treatments stimulated both Cat expression levels and CAT activity in maize and wheat kernels.[27] In another study, there was no correlation between cell growth and the levels of CAT, or glutathione peroxidase (GPx) in various pancreatic cancer cell lines (BxPC-3, MIA PaCa-2, and AsPC-1).[20]
Ji et al. reported that the level of ROS after treatment with different doses of juglone was higher than the control group in SGC-7901 cells.[10] Cullen et al. demonstrated that there were similar levels of CAT immunoreactive protein among all of the pancreatic cancer cell lines; so CAT activity did not correlate well with CAT immunoreactive protein and GPx activity did not correlate significantly with GPx immunoreactive protein.[20]
GSH is found in extracellular spaces and intracellular GSH, GPx, and GR are mainly in the cytosol and mitochondrial compartments.[28] Ourique et al. demonstrated that juglone and Q7 in combination with ascorbate caused antiproliferative effect in tumor cells in vivo by leading to apoptosis and cell cycle control regulated with levels of GSH.[29] Furthermore, an increase in GSH levels was connected with increased ROS production. In GSH pre applied cancer cells, it is explained that the antiproliferative effect of juglone caused GSH utilization.
ROS may show pro-cancer or anticancer effects according to the treated substance concentration and duration of the substance administration in cancer cells. Therefore, it may have opposite effects on the enzymes that regulate the antioxidant system. In our study, it was observed that the application of juglone exposed in different hours caused variability in the antioxidant activity of cells.



Our study showed the critical role of redox homeostasis in the regulation of cancer cell proliferation and cell death and supported that it is possible to kill cancer cells through a ROS-mediated mechanism. Since ROS stress is prevalent in cancer cells, activated different signal pathway, the ROS-based approach may have extensive therapeutic applications.
Subscribe now for latest articles and news.