

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2020.v06i03.007
Year: 2020, Volume: 6, Issue: 3, Pages: 40-45
Original Article
Barnali Mitra1, Anuj Bhatnagar2, Sanjay Kumar3, Ran Singh4, Gautam Kumar Singh5, Debdeep Mitra6
1Associate Professor, Department of Pediatrics, Base Hospital Delhi Cantt, New Delhi, India,
2Associate Professor, Department of Dermatology, Command Hospital (Air Force), Bangalore, Karnataka, India,
3Assistant Professor, Department of ENT, AFCME, New Delhi, India,
4Assistant Professor, Department of Medicine, AFCME, New Delhi, India,
5Associate Professor, Department of Dermatology, Base Hospital Delhi Cantt, New Delhi, India,
6Professor, Department of Dermatology, Base Hospital Delhi Cantt, New Delhi, India
Address for correspondence:
Ran Singh, Department of Internal Medicine, AFCME, New Delhi, India. Phone: 9871637738. E-mail: [email protected]
Background: Pityriasis alba (PA) is a common, benign dermatosis, frequently encountered in children and adolescents. It is clinically characterized by asymptomatic to slightly pruritic, ill-defined hypomelanotic macules predominantly seen over face, arms, and upper trunk. Topical pimecrolimus and calcitriol are promising treatment alternatives for treatment of PA. At present, there is no comparative trial to evaluate the efficacy of 0.0003% calcitriol ointment and 1% pimecrolimus cream vis-a-vis placebo for the management of PA in Indian skin.
Methods:A total of 472 PA lesions, in 226 patients between the age group of 4 and 16 years, were selected over a period of 2 years. They were assigned to three groups to evaluate 157 lesions in pimecrolimus group, 157 lesions in calcitriol group, and 158 lesions in placebo group using petrolatum. Patients were randomly assigned in each group in a double-blinded manner and were instructed to apply the medication twice a day. They were assessed on the basis of digital photographic registration and independent observer analysis at baseline, 2, 4, 6, and 8-week interval for clinical improvement.
Results: Reduction from mean baseline in PA lesions in calcitriol and pimecrolimus group was higher than placebo group from weeks 2 to 8. Both pimecrolimus and calcitriol had significant improvement in the lesions and there was no statistical difference in their results; however, the outcome was significantly better than the placebo.
Conclusions: Among the three-group studied, both topical calcitriol and pimecrolimus showed significant improvement as compared to petrolatum used as a placebo.
KEY WORDS:Calcitriol, pimecrolimus, pityriasis alba, calcineurin inhibitors, Vitamin D analogs.
Pityriasis alba (PA) is a common idiopathic dermatosis more commonly seen in children and preadolescent children and has no gender predilection.[1] The term PA is derived from the Greek word pityron signifying bran or bran like and Latin word albus meaning white. This entity is addressed by many synonyms such as furfuraceous impetigo, erythema streptogenes, and pityriasis streptogenes in the literature.[2] PA is widely regarded as an idiopathic localized hypopigmentary disorder clinically presenting as whitish, round to oval macule or patch which is mostly asymptomatic but may have mild associated itching. The lesions of PA are predominantly located on face, but other areas of the body such as chest, neck, or trunk may be involved.[3] The distribution is mostly symmetrical, though asymmetry can also be seen. In early stages, the patches present as an erythematous macule with slightly elevated borders and may last for weeks. In intermediate stages, the patch has a smooth and scaly layer. A mature PA lesion is hypopigmented, 0.5 to 5 cm in diameter, well defined borders and loosely adherent scales. It is generally at this stage, patients present due to cosmetic concern. In most of the cases, PA clears off by puberty.[4]
The cases of PA are mostly clustered in poor socioeconomic condition but has a worldwide distribution.[5] Multiple factors are proposed in the etiopathogenesis of PA such as sun exposure, poor skin hydration, frequent bathing, xerosis, atopy, parasitic infection, anemia, mineral deficiency, and neglect of skin hygiene.[6]
There are two clinical variants of classical PA: Endemic PA, effecting children from poor economic conditions, and atopy-related PA. Another variant of PA, extensive PA (EPA) is more common in adults, has a generalized and symmetric distribution mainly on trunk and generally has no history of underlying atopy and erythema. However, both atopic and idiopathic variant of EPA are noticed. Both classical PA and EPA have reduced melanocytes and melanosomes. The end result of the process is reduced melanin resulting in hypopigmented patches.
PA typically follows a chronic relapsing remitting course; however, the lesion usually resolves without treatment in most of the cases. In patients with atopic dermatitis, the disease usually has a progressive course. Topical use of corticosteroids, sunscreens, and antiseptics forms the mainstay of treatment.[7] Classical PA usually responds well with lubricants and emollients. The current standard management of PA is use of mild-to- moderate topical steroids. Topical non-halogenated corticosteroids have been used on face, while more potent steroids can be prescribed for non-facial skin involvement such as trunk, chest, and back. Long-term use of topical steroids can result in skin atrophy and hypopigmentation, especially on the facial skin.[8]
Topical calcineurin inhibitors and Vitamin D analogs like calcitriol are newer promising treatment options, though not widely studied. The lack of cutaneous adverse effects and relatively better long- term safety profile makes them a better option, especially in pediatric age group and on facial skin. In the past, pimecrolimus and tacrolimus have shown good results in PA with underlying atopic dermatitis and are preferred for facial PA. Calcitriol has been tried in the management of endemic PA, with satisfactory response. Calcitriol has an edge over Pimecrolimus for management of PA, as the risk of erythema, stinging, and long-term side effects like carcinogenesis is not associated with it.
The study was carried out at a tertiary care hospital in north eastern India over a period of 2 years. The study protocol was approved by the Institutional Ethics Committee. Written informed consent was taken from patients or their parents. Patients comprised both genders between the age group of 4 and 16 years, attending our outpatient clinic. A total of 226 patients with 472 PA patches, distributed symmetrically on face between the size of 2 and 7 cm2, were taken.
Patients with other dermatoses mimicking PA were excluded based on clinical examination, woods lamp, and fungal culture. Patients with concomitant atopic dermatitis, any systemic disease, or medication intake in last 4 weeks were excluded from the study. Although PA is one diagnostic criteria for atopic dermatitis, we did not include diagnosed patients of atopy as they might be on systemic immunosuppressives and which might alter the results; hence, patients with just PA as their initial presentation were included in the study. Target lesions were identified and patients were advised to have timely follow up at 2, 4, 6, and 8 weeks. They were randomly divided into three groups to receive 0.0003% calcitriol, 1% pimecrolimus, and petrolatum on the target lesion. The medication was dispensed in a similar container for all the three groups. Patients were instructed to stop sunscreen application and to apply the topical creams twice daily. Any adverse effects such as redness, stinging, and burning were to be noted and reported on subsequent follow-up.
Patients were examined on 2, 4, 6, and 8 weeks duration for clinical improvement. The primary outcome to be assessed was reduction in the target area. Clinical outcome was assessed based on digital photographic record (frontal, right, and left view). All the images were analyzed using a software ImageJ v1.4, to quantify the size of the target lesion. All the lesions were given a square centimeter score at the time of first examination and the second score was obtained at the end of 8 weeks. An independent observer performed a Physical Global Assessment (PGA), which gave the scored as poor (0–20%), mild (20–50%), good (50– 75%), and excellent (>75%). Any lesion beyond 1.5 cm of target lesion was considered as perilesional.
This examination was conducted at every follow-up visit.
We calculated that a sample size of 93 lesions per intervention would detect a difference in the PA improved area between the active treatments and control of 25% (i.e., 60% in treatments groups and 35% for the placebo), at 95% confidence interval, and two tails, α of 0.05 and β of 0.8. However, a total of 226 patients with total of 472 lesions were randomly divided into three groups with 157 PA lesions to be evaluated in calcitriol and pimecrolimus group each and 158 lesions in placebo group. The data were analyzed using appropriate statistical software. Statistical tests such as Analysis of Variance or ANNOVA were used for finding statistical significance of the results. Turkey range test or Turkey–Kramer method was used for a single- step multiple comparison method for comparing the three variables.
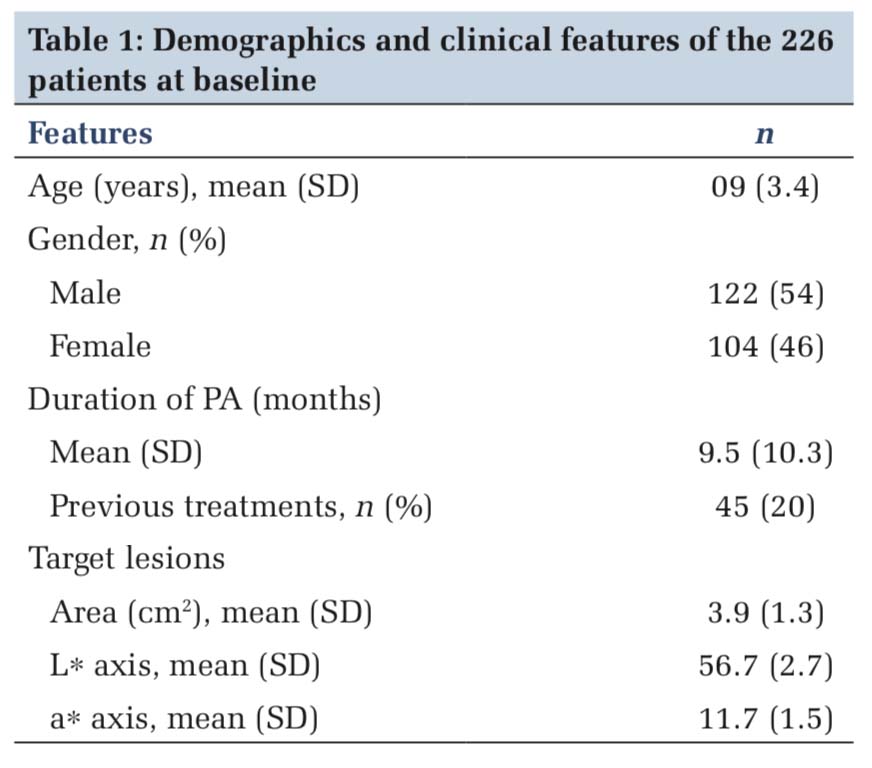
The study started with total 226 patients with 472 PA lesions distributed bilaterally symmetrical on face. Patients of both genders between the age group of 4 and 16 years were taken. Patients were randomly divided into three groups with 157 PA lesions to be evaluated in calcitriol and pimecrolimus group each and 158 lesions in placebo group. At the end of 8-week 126 PA lesions in calcitriol group in 57 patients, 131 lesions in pimecrolimus group in 61 patients and 129 PA lesions in placebo group in 53 patients could be evaluated. Hence, the study was conducted on 171 patients with total 386 PA lesions. The mean age of occurrence was 09 ± 3.4 years. The demographics, mean duration, mean affected area, and measurements are depicted in Table 1.
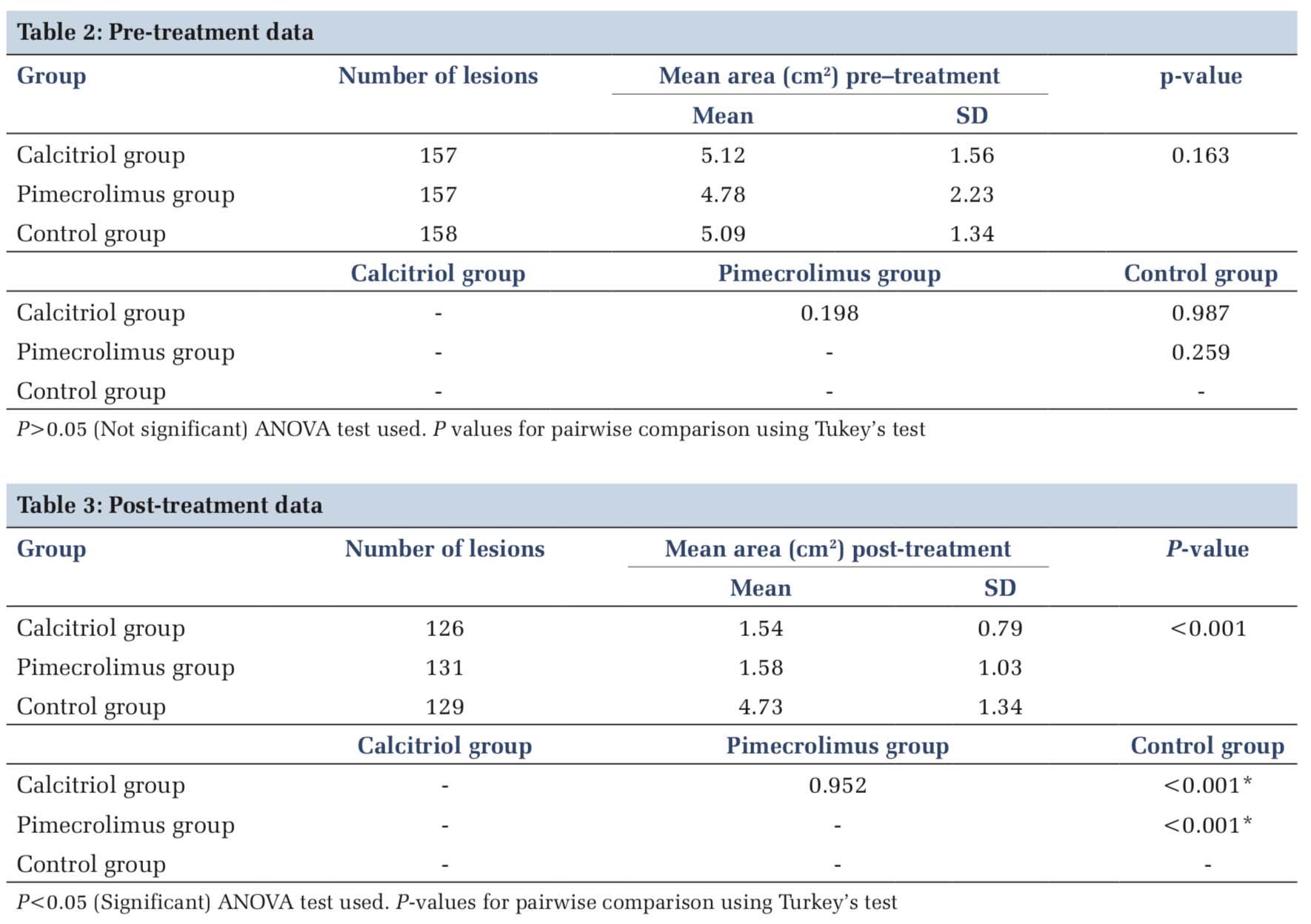
At the end of 8 weeks, we observed that clinical improvement expressed as mean +SD percentage change was higher from base line in calcitriol and pimecrolimus group than the placebo group treated with petrolatum. The statistical analysis is shown in Table 2 as the pre-treatment data and Table 3 documents the post-treatment data. The mean area reduction in the calcitriol group at the end of study period was from 5.12 to 1.54. This was not statistically significant from the pimecrolimus group which recorded a difference of 4.78 versus 1.58 as pre-treatment and post-treatment results. However, both medicines were statistically better than the placebo which just showed an improvement of 5.09 versus 4.73. The graphical data are shown in Figure 1.
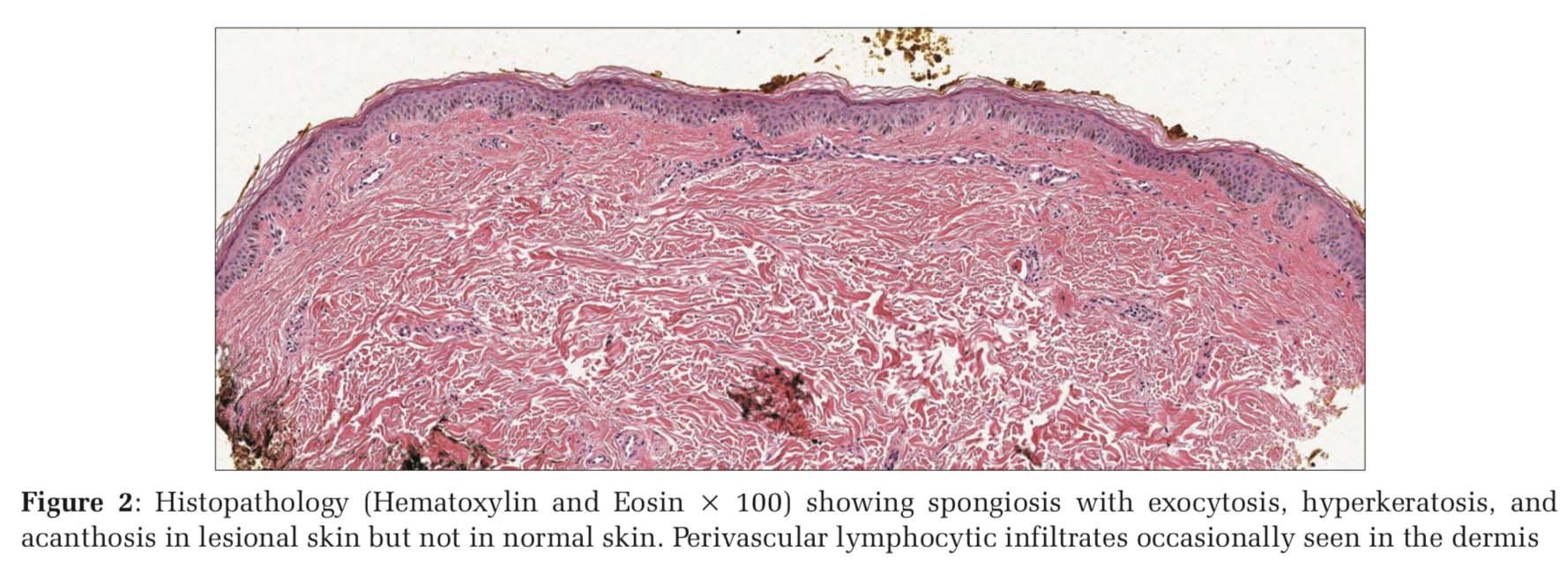
The histopathology of PA lesions showed spongiosis with exocytosis, epidermal hyperkeratosis, and acanthosis which were prominent in lesional skin but not in normal skin. In the dermis, perivascular lymphocytic infiltrates were occasionally seen [Figure 2).
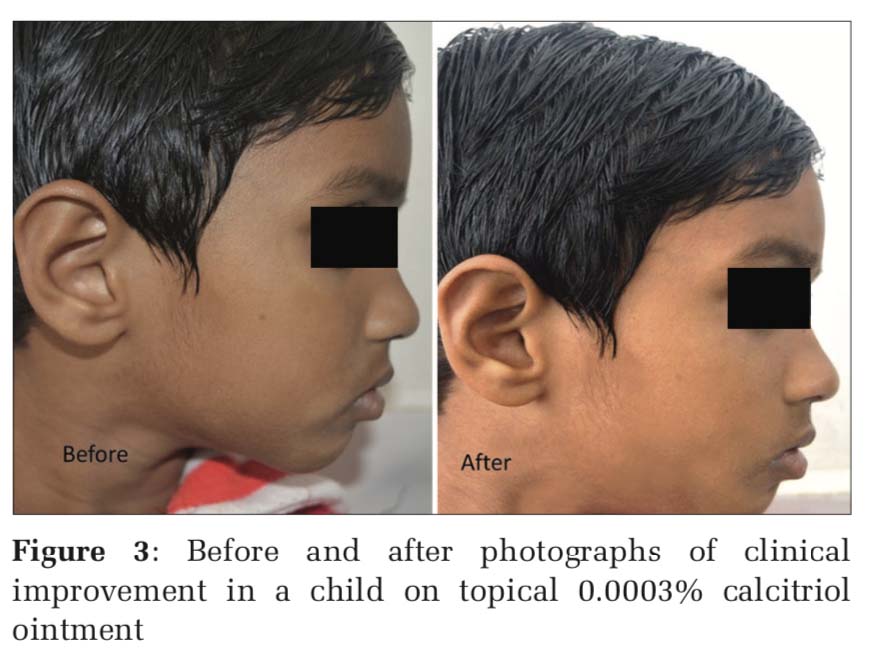
Figure 3 shows before and after images of clinical improvement in a child on topical 0.0003% calcitriol ointment and Figure 4 shows improvement with 1% pimecrolimus cream application.
PA is an idiopathic inflammatory dermatosis, predominantly effecting face of children, and preadolescent age group. The condition has a complex pathogenesis and is difficult to treat, especially in patients with darker skin type where it is easier to notice hypopigmentation.[9] Since decades topical mild-to-moderate steroids have been used in the management of PA; however, a chronic relapsing nature of the disease, involvement of face resulting in atrophy, and hypopigmentation has led to the search of newer topical modalities for the management of PA. Sun protection, humectants, and antiseptics have been used for managing PA for decades, with not very significant change.
1% Pimecrolimus cream is a topical calcineurin inhibitor with anti-inflammatory properties. It prevents T-cell activation and down-regulates T cell production of Th1 and Th2 type cytokines, inhibits T-cell proliferation after antigen stimulation, and reduces mast cell degranulation.[10,11] Pimecrolimus is extensively being utilized in atopic dermatitis with a good efficacy and safety profile record as it lacks the cutaneous adverse effects associated with long-term use of topical steroids. A single case report of improvement in PA in a child was reported by Fox et al. in a African child presenting with depigmented patches over face.[12] Another exploratory study done by Fujita et al. on ten patients over 12 weeks found that pimecrolimus is an effective and safe treatment option in the management of PA. The efficacy was assessed by investigator global assessment of disease severity and evaluation of uneven skin color, eczema pruritus, and follicular keratosis. The study found consistent improvement in color, eczema, and follicular keratosis by week 12. Mild greasiness- stickiness was reported by patients initially, which improved as the treatment progressed.[8]
In our study, we found a consistent improvement in the pimecrolimus group based on PGA, which was noticeable as early as 2 weeks of therapy and thereafter consistently improved through next 12 weeks. Only few patients had complained of stinging and burning due to pimecrolimus, which subsided in all the patients over another 2–3 weeks.
Calcitriol (1, 25- dihydroxyvitamin D3) is an edogenenous hormonally active derivative of Vitamin D. Keratinocytes have receptor for calcitriol, which result in modulation of differentiation and inflammation.[13] Calcitriol also has a regulatory role in melanocyte development and melanogenesis. By virtue of its intrinsic property, it also restores the natural barrier of the skin and restores epidermal permeability and anti-microbial barrier through activating the cutaneous Vitamin D pathway.[14]
There was no adverse effect noted in the calcitriol group and the improvement was consistent from 2 to 12 weeks of the therapy.


Our study establishes that pimecrolimus cream 1% and calcitriol 0.0003% offer a promising treatment alternative to especially pediatric age group. There is no statistically significant difference in the efficacy of pimecrolimus and calcitriol, but both the drugs have a definite edge over petrolatum used as placebo. There is no significant disadvantage of either.
The study could not work on the etiopathogenesis of PA, which still remains an enigma and further studies are required to elucidate the cause of this common dermatosis. Furthermore, the patents could not be followed up for a longer period of time to note the relapse of this condition and the long-term side effect of these drugs if any.
Subscribe now for latest articles and news.