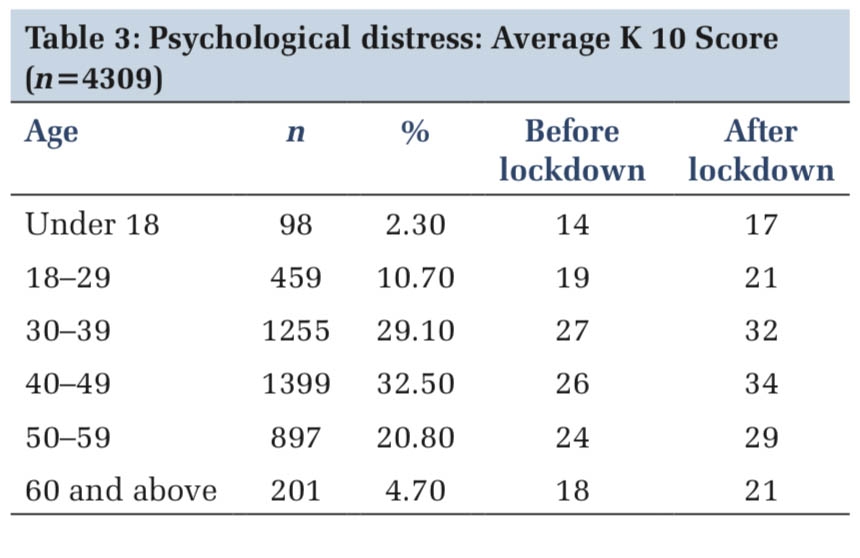
Introduction
This article examines the inflammatory aspect of diabetic kidney disease (DKD) and the potential use of new biomarkers in DKD treatment 1 . It highlights the importance of early identification of inflammatory biomarkers for minimizing consequences and maximizing treatment choices 2 .
Diabetic nephropathy, also known as diabetic kidney disease (DKD), is a unique condition-affecting individuals with both diabetes and chronic kidney disease 3 , altering the kidney's function and altering the body's waste and fluid elimination. The symptoms of this condition are albuminuria, edema, polyuria, nausea, anemia, and hypertension 4 .
The condition escalates the chance of death by 31.1% in individuals who have diabetes, and this risk amplifies as the seriousness of the disease progresses 5 . Timely identification of DKD is essential in order to mitigate the impact on both individuals and the economy. Nevertheless, it often goes unnoticed until significant issues arise.
Implementing early detection methods is a financially efficient strategy to lessen the overall impact. This research focuses on the worldwide incidence, risk factors, and potential early indications of DKD. 6
Epidemiology and Perspectives in DKD
Diabetes mellitus affects 10.5% of people aged 20-79 globally, with 537 million cases reported in 2021 and projected to reach 643 million by 2030 7 . The disease often takes 4-7 years to diagnose, and clinical damage often does not manifest until later 8 .
The development and advancement of this illness include several factors, including hemodynamic, metabolic, and inflammatory processes. The inflammatory axis is becoming a crucial target for therapeutic intervention 9 .
In China, 38.8% of individuals diagnosed with diabetes had DKD, while 2.9% had it in rural dwellers 10 . In India, 34.4% had DKD, and 62.3% had it in a multicenter study 11 . The older population in the eastern Mediterranean has the highest rates of diabetes mellitus and CKD-DM. In Japan, 15.3% of patients with type 2 diabetes had a poor estimated glomerular filtration rate 12 . In the UK, 28% of patients had renal impairment after a 15-year follow-up period. In the United States, 2.2% of the population has DKD, and the prevalence is rising in direct correlation with diabetes occurrence 13 . The research emphasizes that in order to overcome DKD, there is a need for more awareness and preventative efforts.
Diabetic Kidney Disease (DKD) is a prevalent global health issue, with an estimated incidence of 1.75 in people with diabetes 14 . Research conducted in China, India, and the United Arab Emirates reveals varying incidences of DKD.
Pathophysiology of DKD
The etiology of diabetic kidney disease (DKD) involves a multifactorial interaction of inflammatory, hemodynamic, and metabolic variables 15 . Hyperglycemia sets off a series of metabolic reactions that result in endothelial dysfunction, oxidative stress, and damage to the tubules and glomerulus 16 . Tubular dysfunction affects the processes of reabsorption and secretion, whereas renal hyperfiltration and elevated intraglomerular pressure lead to glomerular damage 17 . Renal injury is further aggravated by chronic inflammation and fibrosis, which leads to a steady loss in kidney function 12 . Comprehending these pathophysiological pathways is essential for creating tailored treatments meant to maintain renal function and enhance DKD outcomes.

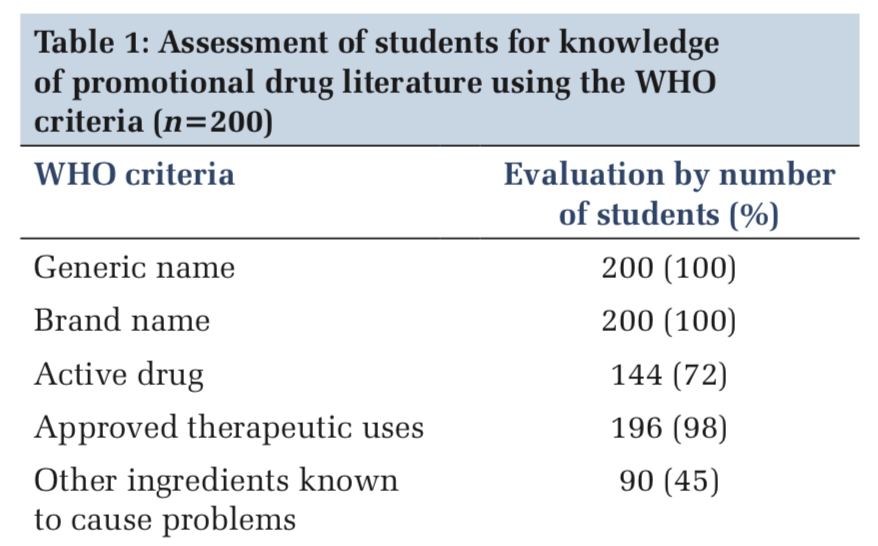
Table 1 provides a comprehensive overview of the various factors that contribute to the development and progression of DKD, emphasizing the need for comprehensive management strategies. 18
|
Sr.No |
Contributors |
Description |
|
1 |
Hyperglycemia |
Elevated blood glucose levels leading to metabolic changes and kidney damage |
|
2 |
Hypertension |
High blood pressure contributing to renal microvascular damage |
|
3 |
Dyslipidemia |
Abnormal lipid metabolism leading to endothelial dysfunction and renal injury |
|
4 |
Genetic Predisposition |
Inherited susceptibility to kidney disease in individuals with diabetes |
|
5 |
Inflammation |
Chronic low-grade inflammation exacerbating renal damage |
|
6 |
Oxidative Stress |
Imbalance between reactive oxygen species (ROS) and antioxidant defenses |
|
7 |
Renin-Angiotensin System Activation |
Over-activation of the renin-angiotensin system leading to vasoconstriction and fibrosis |
|
8 |
Protein Kinase C Activation |
Dysregulation of protein kinase C signaling pathways contributing to renal injury |
|
9 |
Advanced Glycation End Products (AGEs) |
Accumulation of AGEs leading to endothelial dysfunction and inflammation |
|
10 |
Renal Hyperfiltration |
Increased glomerular filtration rate resulting in glomerular damage |
|
11 |
Endothelial Dysfunction |
Impaired endothelial function contributing to vascular pathology |
|
12 |
Podocyte Injury |
Damage to podocytes disrupting glomerular filtration barrier |
|
13 |
Tubulointerstitial Fibrosis |
Accumulation of extracellular matrix proteins leading to renal fibrosis |
|
14 |
Autoimmunity |
Immune-mediated injury to renal tissue in susceptible individuals |
|
15 |
Environmental Factors |
Lifestyle, diet, and other environmental factors influencing disease progression |
The metabolic route encompasses the use of polyol, hexosamine, advanced glycation end products, and protein kinase C 19 . Hyperglycemia leads to kidney injury by forming glycosylated end products, activating protein kinase C, and synthesizing diacylglycerol 20 .
Limitations of Conventional Biomarkers
Albuminuria and a decrease in the estimated glomerular filtration rate (eGFR), which is determined by blood creatinine levels, are diagnostic indications of diabetic kidney disease (DKD). 21 Albuminuria is a reliable biomarker for predicting future progression and evaluating treatment efficacy. Urinary albumin excretion (UAE) is used for risk stratification and measures the extent of albuminuria, making it a crucial tool in diagnosing and managing DKD 22.
Glomerular filtration rate (GFR) is the primary clinical biomarker used to evaluate the prognosis of diabetic kidney disease (DKD) during follow-up. When glomerular filtration rate falls, there is a clear correlation between structural damage and kidney function, but this association is less apparent with mild albuminuria or a slight decline in eGFR 23.
Changes in the present and prior estimated GFRs (eGFRs) are important indicators of how the illness is likely to proceed. The glomerular filtration rate (GFR) is often estimated using the CKD-EPI and MDRD equations. 24 However, it is important to note that these equations have the potential to either underestimate or overestimate the actual GFR. This discrepancy may occur because of compensatory adjustments in the functioning of the remaining nephrons 25. Particularly for those with the common normoalbuminuric phenotype who lack particular therapy options, the diagnostic and follow-up techniques already in use have drawbacks. 26
The research highlights the need for novel biomarkers to improve diagnostic and monitoring methods for diabetic kidney disease, focusing on their effectiveness in diagnosing, monitoring progression, assessing treatment response, and predicting prognosis. 27 The study will investigate the practical application of new biomarkers, as illustrated in Figure 2.
Figure 2 shows tables that provide comprehensive list of overview of conventional biomarkers 28, 29 vs novel biomarkers 30, 31, 32 commonly used in clinical practice to diagnose, monitor, and manage Diabetic Kidney Disease, focusing on kidney function, damage, and overall metabolic control.

Novel Biomarkers in Diabetic Kidney Disease
Early detection is crucial for managing diabetes-related kidney disease, but traditional diagnostic techniques often lead to delayed diagnosis 33. The study of DKD pathophysiology and mechanisms has heightened the importance of discovering novel biomarkers in urine and serum through proteomics and metabolomics.
Advancements in kidney damage knowledge have led to the development of biomarkers that can aid in diagnosing diabetic kidney disease (DKD). These biomarkers, unlike conventional ones like serum creatinine, can enhance the accuracy of DKD assessments, making them more precise tools for evaluating kidney function 34 .
The AKI agreement asserts that biomarkers should not replace conventional testing and clinical evaluations but rather serve as supplementary examinations to identify individuals who can benefit from cardiovascular risk prevention and management strategies 35 .

Biomarkers for the early diagnosis of DKD
Biomarkers like urine albumin excretion rate, UAER, UACR, and eGFR help detect renal damage before clinical signs of diabetic kidney disease (DKD) 6 . New biomarkers like L-FABP, NGAL, and KIM-1 show promise in early detection of renal damage. Integrating these indicators into clinical practice can help mitigate DKD development and related consequences through early intervention and individualized care techniques.
Timely identification and suitable therapies are vital for effectively treating kidney disease (DKD), as they may effectively slow down the course of the illness, enhance life expectancy, and alleviate the physical, emotional, and financial strain associated with it 38 . The absence of diagnostic indicators, such as albuminuria and serum creatinine, poses a hindrance to the timely detection of a condition 39 . Research has shown that albuminuria is an unreliable and insensitive indicator, while serum creatinine does not possess strong predictive capability 40 . Hence, new biomarkers are needed for early diagnosis and treatment of DKD due to their sensitivity and predictability, as existing biomarkers are not very sensitive.

Diagnosis of DKD

Diagnosis of diabetic kidney disease involves assessing urine albumin excretion and glomerular filtration rate (GFR) 44 . Early renal impairment is detected through albuminuria screening, while chronic albuminuria and GFR measurement aid in staging DKD severity 45 . Further diagnostic procedures include serum biomarkers, kidney biopsy, and renal imaging. Early identification and monitoring are crucial for prompt intervention and therapy to prevent DKD from progressing to end-stage renal disease.
Table 2 shows various diagnostic assessments used in the diagnosis of DKD, focusing on indicators of kidney function, damage, and overall metabolic control. These steps help in early detection, monitoring disease progression, and guiding treatment decisions. 46
|
Sr. no |
Diagnostic Step |
Description |
|
A |
Screening |
|
|
1 |
Annual Screening |
All patients with diabetes should undergo annual screening for kidney disease |
|
2 |
Urinary Albumin Excretion |
Measurement of urinary albumin-to-creatinine ratio (UACR) in a spot urine sample. Persistent albuminuria (>30 mg/g) indicates kidney damage 7 |
|
3 |
Glomerular Filtration Rate (GFR) |
Calculation of estimated GFR (eGFR) using equations such as MDRD or CKD-EPI. Decreased eGFR (<60 mL/min/1.73m^2) indicates kidney damage |
|
B |
Diagnostic Confirmation |
|
|
1 |
Confirmation of Albuminuria |
Confirmatory testing with at least two out of three urine samples collected over 3-6 months |
|
2 |
Measurement of Serum Creatinine |
Measurement of serum creatinine levels to estimate GFR and assess kidney function |
|
C |
Additional Tests |
|
|
1 |
Renal Imaging |
Imaging studies such as ultrasound to assess kidney structure and identify anatomical abnormalities |
|
2 |
Serum Biomarkers |
Measurement of serum cystatin C or urinary biomarkers (e.g., KIM-1, NGAL) for additional information about kidney injury and prognosis |
|
3 |
Renal Biopsy |
In select cases, renal biopsy may be indicated to confirm diagnosis and guide treatment decisions |
|
D |
Evaluation of Complications |
|
|
1 |
Cardiovascular Assessment |
Assessment of cardiovascular risk factors (hypertension, dyslipidemia) given the high risk of cardiovascular disease in DKD |
|
2 |
Screening for Other Complications |
Regular screening for other diabetes-related complications such as retinopathy and neuropathy |
|
E |
Monitoring and Follow-up |
|
|
1 |
Regular Monitoring |
Regular monitoring of kidney function (eGFR, UACR), blood pressure, glycemic control, and other relevant parameters to guide treatment and assess disease progression |
|
2 |
Multidisciplinary Care |
Collaborative care involving primary care providers, endocrinologists, nephrologists, and other specialists for comprehensive management of DKD and associated comorbidities |
Diagnosing diabetic kidney disease (DKD) involves UACR measurement and GFR assessment, which should be less than 60 ml/min per 1.73 m2. Even in cases of type 1 and type 2 diabetes, GFR decreases even when the ratio is within permissible limits. It is crucial for diabetics to check their creatinine levels annually. GFR can be calculated using formulas like the MDRD formula and the CKD-EPI equation. The American Diabetes Association recommends screening for nephropathy in all patients with type 2 diabetes and those with type 1 diabetes for more than five years.
|
Sr. no |
Treatment |
Description |
Examples |
|
1 |
Glycemic Control |
Tight glycemic control to delay DKD progression |
Metformin, Insulin, SGLT2 inhibitors |
|
2 |
Blood Pressure Management |
Use of RAAS blockers to reduce proteinuria and slow DKD progression |
ACE Inhibitors (e.g., Lisinopril), ARBs (e.g., Losartan) |
|
3 |
Lipid Management |
Statin therapy to reduce cardiovascular risk in DKD |
Atorvastatin, Rosuvastatin |
|
4 |
Protein Restriction |
Moderate protein restriction to reduce proteinuria |
Dietary counseling |
|
5 |
Sodium Restriction |
Limiting sodium intake to manage hypertension and fluid retention |
Dietary counseling |
|
6 |
RAAS Blockade |
Use of ACE inhibitors or ARBs to preserve renal function |
Enalapril, Irbesartan |
|
7 |
Anticoagulation Therapy |
Consideration of anticoagulation to reduce thromboembolic risk |
Enoxaparin, Apixaban |
|
8 |
Antiplatelet Therapy |
Use of antiplatelet agents to reduce cardiovascular risk |
Aspirin, Clopidogrel |
|
9 |
Lifestyle Modifications |
Promotion of healthy lifestyle behaviors. |
Diet, Exercise, Smoking cessation |
|
10 |
Regular Monitoring |
Monitoring kidney function, blood pressure, and glycemic control. |
eGFR, UACR, Blood pressure |
|
11 |
Referral to Nephrology |
Consultation with nephrologist for comprehensive management. |
Nephrologist appointment |
Conclusion
In recent years, new treatments for DKD have emerged due to ongoing research into potential targets for prediction, follow-up, and prevention. Nevertheless, the integration of these indicators into clinical practice is currently undergoing development. Advancements in the development of new molecules and therapeutic approaches show promise for expanding the options available to doctors for more effective and individualised therapies in the future.
DKD is a medical disaster with high occurrence rates, inadequate detection methods, and a lack of new indicators for early diagnosis. Multinational and multicenter epidemiological investigations are necessary to validate the potential and suitability of novel plasma and urine biomarkers, which are proving effective for timely detection.