

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.v11.i2.25.25
Year: 2025, Volume: 11, Issue: 2, Pages: 223-227
Original Article
K C Abhay1 , B P Preethi2 , R R Chaitali3 , Prathyush Reddy4
1Final year Medical Student, J J M Medical College, MCC B Block, Davangere, 577004, Karnataka, India,
2Professor, Department of Biochemistry, Member & Resource person, Medical Education Unit, J J M Medical College, MCC B Block, Davangere, 577004, Karnataka, India,
3Consultant Neonatologist, Department of Neonatology, J J M Medical College, MCC B Block, Davangere, 577004, Karnataka, India,
4Senior Resident, Pediatrics, J J M Medical College, MCC B Block, Davangere, 577004, Karnataka, India
Address for correspondence:
B P Preethi, Professor, Department of Biochemistry, Member & Resource person, Medical Education Unit, J J M Medical College, MCC B Block, Davangere, 577004, Karnataka, India.
E-mail: [email protected]
Received Date:18 January 2025, Accepted Date:09 March 2025, Published Date:05 September 2025
Introduction: Jaundice is observed during the first week of life in most of the newborns. Milder ones resolve spontaneously while severe ones need phototherapy or exchange transfusion. So, there is a need to screen all newborns for hyperbilirubinemia. Total serum bilirubin measured in the laboratory is the standard protocol followed but is invasive. Transcutaneous bilirubin, measured by a bilirubinometer, is emerging as a promising non-invasive tool. With this context, we evaluated Transcutaneous bilirubin as a screening tool for identifying hyperbilirubinemia among term neonates. Materials and methods: A cross-sectional observation study was conducted among 137 clinically icteric neonates using paired bilirubin measurements, one estimating bilirubin by standard laboratory method and the other measuring Transcutaneous bilirubin measured by a Bilirubinometer. Results: 137 neonates participated in the study. 61% were boys and 39% were girl babies. The mean gestational age was 37.28±1.7 weeks. The average birth weight of the study subjects was 2.7±0.3 kg. The mean bilirubin concentration measured by TCB was 9.07±3.6 and that estimated in the laboratory, TSB was 10.32±4.1. A strong linear statistically significant correlation r=0.849 with p<0.001 was observed between TSB and TCB. Bland Altman analysis revealed a difference of 1.25mg/dl between TSB and TCB with the majority of data points falling within ±1.96 times of SD indicating strong agreement between TSB and TCB. Conclusion: Strong correlation and agreement between TSB and TCB support the use of TCB as an effective screening tool for identifying hyperbilirubinemia among term neonates.
Keywords
Hyperbilirubinemia, Transcutaneous bilirubin, Total serum bilirubin, Term neonates, Screening tool, RESEA
Jaundice is observed during the first of life in approximately 60% of term and 80% of preterm babies 1. Most often this is physiologic jaundice which refers to the occurrence of jaundice in a neonate in whom the bilirubin rate of rise does not cross percentile curves and in whom peak bilirubin levels are below the 95th percentile for age. Around 2% of affected babies are at risk of severe hyperbilirubinemia, where bilirubin levels are above the 95th percentile for age. This severe hyperbilirubinemia is a risk factor for bilirubin encephalopathy which is a preventable cause of death and long-term disability. 2 An age-specific nomogram of total serum bilirubin is used as a deciding factor for initiating therapy for hyperbilirubinemia. 3 Sampling for estimation of total serum bilirubin is a painful procedure predisposing neonates to nosocomial infections and requires a skilled phlebotomist. Exposure of the sample container to ambient light degrades bilirubin resulting in false low values. Moreover, measurement of total serum bilirubin in the laboratory is time- consuming and there are chances of delay in commencing phototherapy. 4 Transcutaneous bilirubin measured using a bilirubin meter is emerging as a promising tool for identifying hyperbilirubinemia. These devices use multi-wavelength spectral reflectance from the skin surface to quantify bilirubin thus avoiding the need for blood sampling. 5 The present study was conducted to evaluate the correlation between bilirubin concentration measured using a transcutaneous bilirubin meter and bilirubin estimated by the standard laboratory method.
This was a cross-sectional observational study conducted between April and October 2022 among 137 neonates admitted to Bapuji Hospital of JJM Medical College, Davangere, Karnataka after obtaining Institutional Ethical Committee clearance (ref no: JJMMC/IEC-04-2022) and written informed consent from parents of neonates. Inclusion criteria were as follows: neonates of both gender with birth weight ≥2.5Kgs, delivered either by vaginal route or by cesarean section after completion of 35 weeks of gestation and clinically diagnosed jaundice babies with yellowish discoloration of skin and conjunctiva after 24 hours of delivery who were not on phototherapy were included as study subjects. Babies at high risk of neonatal hyperbilirubinemia including preterm, low birth weight, G6PD deficiency, ABO incompatibility, babies having clinical jaundice within 24 hours of delivery, and babies on phototherapy and exchange transfusion were excluded. A sample size6 of 137 concomitant assays was calculated based on an estimated sensitivity of 90% with the desired absolute difference between sample and population sensitivity of 10% and with type 1 error of 0.05.
Expected sensitivity: 0.90
Expected specificity: 0.85
Prevalence of disease (p): 0.6 or 60%
Precision (± expected) :0.10
Confidence level 100 (1 - α): 95%
Expected dropout rate: 10 %
Sample size for sensitivity, nsen = 58
Sample size for specificity, nspec = 123
Final sample size (largest), n = 123
Final sample size (with 10% dropout), ndrop = 137
Data collection: Bilirubin measurement using (transcutaneous bilirubin meter) TCB and by estimation of (total serum bilirubin) TSB in hospital central laboratory was done once for each neonate with a maximum interval of 30 minutes between them. Venipuncture was done and samples were protected from ambient light and transported to the laboratory. TSB measurement was done using the modified Diazo method 7 on the ROCHE INTEGRA 400 autoanalyzer. TCB measurement 8 was done using a single Drager device by the same trained personnel. TCB works by directing light into the skin and measuring the intensity of the reflected wavelength of light. The device was placed on the neonate’s forehead capturing three consecutive TCB readings and the mean of the three readings was used for analysis.
Statistical analysis was performed using SPSS version 20. Qualitative/categorical variable like gender is expressed as absolute number and frequency. Quantitative/continuous variables like age, birthweight, TCB, and TSB are expressed as mean ± standard deviation. The association between TCB and TSB was determined using Pearson’s correlation. To assess the agreement between TCB and TSB, the Bland-Altman graph was constructed. Sensitivity, specificity, positive and negative predictive values for TCB were calculated.
137 neonates participated in the study. 61% were boys and 39% were girl babies. The mean gestational age was 37.28±1.7 weeks. The average birth weight of the study subjects was 2.7±0.3 kg. The mean bilirubin concentration measured by TCB was 9.07±3.6 and that estimated in laboratory TSB was 10.32±4.1 (Table 1).
|
|
Mean |
Median |
SD |
Minimum |
Maximum |
|
Gestational age (weeks) |
38.66 |
|
2.6 |
35 |
40 |
|
Birthweight (kgs) |
2.78 |
|
0.5 |
2.5 |
3.5 |
|
TSB (mg/dl) |
10.32 |
10.2 |
4.1 |
3.6 |
22 |
|
TCB (mg/dl) |
9.07 |
8.5 |
3.6 |
1.2 |
18 |
TSB = Total Serum Bilirubin; TCB = Trans Cutaneous Bilirubin
TSB values ranged from 3.6 to 22mg/dl. 75% of neonates had bilirubin values > 95th percentile for age and were suffering from severe hyperbilirubinemia requiring treatment, 4% of babies had bilirubin values < 75th percentile for age with no significant hyperbilirubinemia, and 6% of neonates with levels between 75th percentile and 95th percentile for age accounting to be in intermediate risk for hyperbilirubinemia requiring frequent monitoring (Table 2).
|
|
TSB ≥ 95 th percentile |
TSB ≤ 95 th percentile |
|
|
TCB ≥ 75th percentile |
(103) 75% |
(9) 6% |
(112) 81% |
|
TCB ≤ 75th percentile |
(5) 4% |
(20) 15% |
(25) 19% |
|
Total |
(108) 79% |
(29) 21% |
(137) 100% |
TSB = Total Serum Bilirubin; TCB= Trans Cutaneous Bilirubin
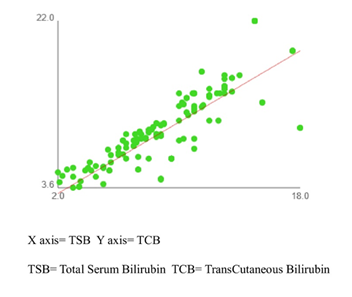
The scatter plot of TSB vs TCB suggests a strong positive correlation (Figure 1). Pearson’s correlation coefficient, r= 0.849 with hypothesis test of correlation with p <0.001 indicates a strong linear association between TSB and TCB. Simple linear regression calculated for STB = 0.9762 TCB +1.466 is used for the prediction of STB from TCB with a high degree of accuracy.
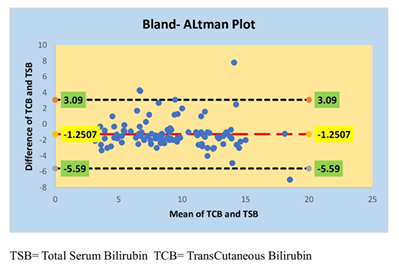
Agreement between TSB and TCB is assessed by the construction of Bland-Altman plot. The baseline indicates that the difference of 1.25 mg/dl between the average of two variables with TSB is higher than TCB. It is observed that the majority of data points fall within ±1.96 times of Standard Deviation of difference between TSB and TCB values suggesting a strong agreement between TSB and TCB (Figure 2).
Diagnostic accuracy analysis for TCB reveals a sensitivity of 95.37% and a positive predictive value of 91.96% in identifying hyperbilirubinemia, a specificity of 66.67%, and a negative predictive value of 78.26% in ruling out hyperbilirubinemia (Table 3).
|
|
TSB ≥ 95 th percentile |
TSB ≤ 95 th percentile |
|
|
TCB ≥ 75th percentile |
103 True positives |
9 False positives |
112 |
|
TCB ≤ 75th percentile |
5 False negatives |
20 True negatives |
25 |
|
Total |
108 |
29 |
137 |
TSB = Total Serum Bilirubin; TCB= Trans Cutaneous Bilirubin
In newborns, hyperbilirubinemia becomes clinically apparent as jaundice characterized by yellowish discoloration of skin and sclera. Hyperbilirubinemia occurs when there occurs an imbalance between bilirubin production and excretion. Most cases of neonatal jaundice are mild and resolve spontaneously, some require phototherapy, and few may require exchange transfusion. There is a need to identify neonates with hyperbilirubinemia requiring therapy. In the present study, bilirubin measurement by transcutaneous bilirubin meter which is a non-invasive POCT device was evaluated as a screening tool for identifying hyperbilirubinemia among clinically icteric 137-term neonates on the third day of their life as the rate of rise of bilirubin is specific to postnatal age.
The mean bilirubin concentration measured by TCB was 9.07±3.6 and that estimated in the laboratory, TSB was 10.32±4.1. The difference between the means of these two variables was 1.2 mg/dl with a 95% confidence interval of 3.11 and -5.61. Correlation between TCB and TSB was found to be linear and statistically significant with r=0.849, p<0.001. Our observations are in alignment with that of Khan et al 9 who reported a correlation of 0.82 and with that of Engle et al 10 and Mandal et al 11 who reported a much higher correlation value of 0.9
Bland and Altman 12 have suggested that correlation could be a poor indicator of agreement between two clinical/laboratory tests and a far better measure of the agreement would be the plot of the difference of values obtained by two methods vis-à-vis the means of values. Bland Altman analysis revealed that the baseline obtained indicates a difference of 1.25 mg/dl between the average of TCB and TSB with TCB being lower than TSB. The majority of data points fall within ±1.96 times of standard deviation reinforcing the presence of strong agreement between TCB and TSB. Our observations of Bland Altman analysis are per a study conducted by Mandal et al who have reported a baseline with a difference of 1.20 mg/dl between TCB and TSB. 11
A high sensitivity of 95.37% and high positive predictive value of 91.96% for TCB levels more than 75th percentile for age was observed among our study participants suggesting the accuracy of TCB in identifying hyperbilirubinemia among term neonates. Our results of TCB measurement are comparable to those of Mandal et al 11 who reported absolute sensitivity with a PPV of 61.2% and Liete et al 13 who reported a sensitivity of 88.2% and PPV of 78.9%. We noted a slightly lower specificity of 66.67% with a negative predictive value of 78.26% for TCB in ruling out hyperbilirubinemia. Low specificity can be attributed to the inclusion of neonates with varying degrees of neonatal jaundice. Even though specificity was low, TCB was able to identify a sizeable group of neonates who did not need invasive testing for hyperbilirubinemia. Our results are in agreement with that of Gunaseelan et al 14 who had prospectively evaluated 400 newborns for hyperbilirubinemia using TCB and have concluded that TCB correlates closely with TSB among neonates born after 35 weeks of gestation even with a low specificity of 56% due to inclusion of clinically icteric neonates.
Our observations strongly suggest that TCB measurement can be used as a first-line screening tool for identifying hyperbilirubinemia among term neonates when TCB values are more than the 75th percentile for age. Newborns with TCB less than the 75th percentile for age, indicate that these babies are at low risk of developing clinically significant hyperbilirubinemia eliminating the need for further testing.
Strength of our study: TCB measurements were done by a single trained person with a single instrument. Paired measurements for TCB and TSB within 30mins difference, were undertaken for all the enrolled neonates on their third day of life.
Limitations: The effect of low birth weight, phototherapy, and skin color on TCB were not evaluated and this provides scope for further studies. TCB accuracy is reduced with exposure of the infant to sunlight or phototherapy. TCB underestimates bilirubin when levels are above 16 mg/dl.
Results of our study support the use of transcutaneous bilirubin measurement using Bilirubinometer as a safe, rapid, non-invasive, point-of-care testing device for screening and identifying hyperbilirubinemia among term neonates.
Acknowledgments: Authors express humble gratitude to all the neonates and their parents for participating in this ICMR STS 2022 project. We also extend our sincere thanks to the NICU team for providing their full cooperation in completing this project.
Conflict of interest: None
Funding: None
Subscribe now for latest articles and news.