

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.v11.i2.25.102
Year: 2025, Volume: 11, Issue: 3, Pages: 237-243
Original Article
Hemlata Rathore1 , Vinita Ailani2 , Rachit Sharma3 , Brijesh Rathore4 , Seema Singh5
1Ph.D. Scholar, Department of Physiology, National Institute of Medical Sciences & Research, Nims University, Jaipur, Rajasthan, India,
2Professor, Department of Physiology, National Institute of Medical Sciences & Research, Nims University, Jaipur, Rajasthan, India,
3Department of Pulmonary Medicine, Era’s Lucknow Medical College & Hospital, Lucknow, Uttar Pradesh, India,
4Department of Biochemistry, Era’s Lucknow Medical College & Hospital, Lucknow, Uttar Pradesh, India,
5Department of Physiology, Era’s Lucknow Medical College & Hospital, Lucknow, Uttar Pradesh, India
Address for correspondence:
Vinita Ailani, Professor, Department of Physiology, National Institute of Medical Sciences & Research, Nims University, Jaipur, Rajasthan, India.
E-mail: [email protected]
Received Date:10 March 2025, Accepted Date:07 June 2025, Published Date:11 September 2025
Objective: Type 2 diabetes mellitus (T2DM) is a multifactorial non-communicable disease that is characterized by insulin resistance and chronic sub-clinical inflammation. IL-1β has been implicated in metabolic processes including insulin secretion and b-cell apoptosis. Increased levels of pro-inflammatory markers such as Interleukin-1β (IL-1β), Tumor Necrosis Factor-α and adipokines has been reported in T2DM patients. Recent studies have demonstrated a positive correlation between Interleukins and lung function impairment in T2DM patients. The objective of the study was to find association of IL-1β and pulmonary function in T2DM patients. Material and Methods: We conducted a cross-sectional study among T2DM patients visiting the OPD of Pulmonary Medicine. T2DM patients aged between 40 to 65 years of either sex were recruited. Biochemical investigations and spirometry were performed. Results: We recruited 340 T2DM patients (162 males and 178 females) for the present study. Biochemical analysis reported 78 subjects with uncontrolled diabetes and 262 subjects with controlled diabetes. Spirometry results revealed significant change (p<0.001) in mean FEV1, mean FVC values and Mean FEV1/FVC ratio (%) in uncontrolled diabetics (2.21±0.57, 3.50±0.48, 62.69±12.18 resp.) as compared to controlled diabetics (2.47±0.42, 3.68±0.37, 67.01±8.43 resp.). Obstructive and restrictive lung impairment was found more in uncontrolled diabetics as compared to controlled diabetics. IL-1β levels were significantly increased (p<0.001, t=21.76) in uncontrolled diabetics (55.23±9.11) in comparison with controlled diabetics (25.39±11.04). Conclusion: Results suggests a significant (p<0.001, r2=0.825) association of IL-1β with pulmonary functions in T2DM patients.
Keywords: Type 2 Diabetes Mellitus, Pulmonary function test, Interleukin-1β, Spirometry
The global diabetes patients are increasing rapidly. In 2000, the International Diabetes Federation (IDF) recorded 151 million cases worldwide. By 2022, that figure had nearly doubled to 285 million, and projections suggest that, at the current rate, approximately 500 million people could be living with diabetes within the next decade. The long-term complications of diabetes severely diminish patients' quality of life, placing a substantial economic burden on both families and individuals 1 .
Studies have demonstrated the decreased pulmonary function (in comparison with normal population-predicted values) in patients with type 2 diabetes mellitus 2 . An Indian study demonstrated that patients with Type 2 Diabetes Mellitus (T2DM) and inadequate control had lower FVC and FEV1 than predicted and then those of subjects with adequate control 3 . Several reports are available indicating an association between lower values of Pulmonary Function Tests and the incidence of T2DM in middle-aged men 4 .
Various factors play a role in the pathogenesis of T2DM, which accounts for more than 90% of the diabetes cases 5 . Previous studies suggest that inflammation could be a potential cause of T2DM and other obesity-associated diseases 6 . Inflammation is an important biological immune defence mechanism 7 . In human, inflammation plays a dual role in various disease. Early inflammatory responses may promote tissue repair. However, severe inflammation can lead to tissue damage 8 . Chronic inflammation is recognized as a key factor contributing to both insulin resistance and the impaired function of beta-cells in T2DM patients 9 . The inflammatory cytokines such as tumour necrosis factor-alpha (TNFα), and interleukins (ILs) can promote the occurrence and development of T2DM and vascular atherosclerosis 10 .
A growing body of research confirms that persistent systemic or localized inflammation is an important factor in the progression of chronic non-infectious diseases, including diabetes and its serious complications such as diabetic kidney disease (DKD) and atherosclerosis 11, 12 . Interleukin-1 beta (IL-1β) plays a central role in the pathogenesis of a wide range of pulmonary inflammatory disorders 13 . IL-1β is a well-established mediator of beta-cell dysfunction and apoptosis, and its effects are amplified by TNF-α and Interferon gamma (IFN-γ) 14 .
A meta-analysis review, using both random and fixed effects models, revealed no statistically significant difference in IL-1β levels between individuals with T2DM and healthy controls 15 . The inflammatory response in COPD, as measured by IL-1β, IL-4, IL-8, TNF-α, and IFN-γ levels, has been directly influenced by the degree of bronchial obstruction and the overall progression of the disease 16 .
This study aims to investigate the association of IL-1β and pulmonary function in T2DM patients.
This prospective, cross-sectional study was undertaken at Era’s Lucknow Medical College and Hospital, Lucknow. The study was carried out in the Department of Physiology in collaboration with the Department of Pulmonary Medicine. Ethical approval for the study was granted by the Institutional Ethics Committee prior to commencement vide letter no NIMSUR/IEC/2023/506 dated 06.03.2023.
Type 2 Diabetes Mellitus patients, attending the OPD of Pulmonary Medicine, Era's Lucknow Medical College, Lucknow, fulfilling the criteria were recruited. Informed consent was obtained from all study participants.
Patients of type 2 diabetes mellitus aged between 40 to 65 years of either sex.
T2DM Patients for more than six months duration.
Patients willing to give informed written consent.
Subjects with current smoking habit or a smoking history.
Those diagnosed to have any acute or chronic respiratory disease such as emphysema, cystic fibrosis, asthma etc.
Pregnant women.
Subject having any other endocrine disorders e.g. Hypo and Hyperthyroidism, Cushing’s syndrome, Grave’s disease, Addison’s disease etc.
Subjects in any occupation affecting pulmonary functions.
Type 2 Diabetes Mellitus Patients were divided into controlled and uncontrolled as per American Diabetes Association criteria (2013) 17 . Patients with HbA1C ≤ 7% were considered controlled and with HbA1C > 7% were considered uncontrolled diabetic.
3ml blood was drawn from all the study participants for cytokine analysis. Interleukin-1β was quantified using enzyme-linked immunosorbent assay (ELISA) kit. The assay procedure was performed according to the instructions provided in the technical bulletin supplied with the kit (R&D Systems, Minneapolis, MN, USA).
Spirometry was performed in all study participants. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the ratio of the forced expiratory volume in 1 second to the forced vital capacity in percentage (FEV1/FVC%) were measured using the Medikro Spirostar (M9479) Finland Machine. A minimum of three performances were recorded. FEV1, FVC and FEV1/FVC% readings were noted. All the readings were interpreted according to ATS/ERS standard 2019 18 .
After the collection of data, statistical analysis was performed using SPSS version 18. Quantitative parametric data are expressed as mean ± SD. Qualitative data are expressed as number and percent of total. Comparative analysis was done using one-way ANOVA. Correlations were done with Pearson’s correlation coefficient. P value less than 0.05 was considered significant.
We conducted the study by recruiting 340 T2DM patients. Of the total T2DM patients, males were 162 (47.65%) and females were 178 (52.35%), indicating female predominance. Patients were categorised in uncontrolled and controlled diabetics depending on the HbA1C value and fasting blood glucose levels. A total of 78 patients had uncontrolled diabetes, compared to 262 patients with controlled diabetes.
Among 78 uncontrolled diabetics, 46.2% were male and 53.8% were female, while among 262 controlled diabetics, 48.1% were male and 51.9% were female. A higher proportion of females was observed in both controlled and uncontrolled T2DM groups compared to males i.e, uncontrolled diabetics (53.8%) as well controlled diabetics (51.9%), this increase was observed statistically non-significant (c2=0.132, p<0.716) (Table 1).
|
SN |
Characteristic/parameter |
Uncontrolled diabetes (n=78) |
Controlled diabetes (n=262) |
Statistical significance |
|
1. |
Age (years) Mean±SD (Range) |
56.24±6.87 (40-65) |
51.59±7.15 (40-65) |
t=5.280; p<0.001 |
|
2. |
Gender |
|
|
|
|
|
Male Female |
36 (46.2%) 42 (53.8%) |
126 (48.1%) 136 (51.9%) |
c2=0.132; p=0.716 |
|
3 |
FEV1 (L) (Mean ±SD) |
2.21±0.57 |
2.47±0.42 |
t=4.493; p<0.001 |
|
4 |
FVC (L) (Mean ±SD) |
3.50±0.48 |
3.68±0.37 |
t=3.522; p<0.001 |
|
5 |
FEV1/FVC ratio (%) (Mean ±SD) |
62.69±12.18 |
67.01±8.43 |
t=3.555; p<0.001 |
|
6 |
IL-1β (pg/ml) (Mean ±SD) |
55.23±9.11 |
25.39±11.04 |
t=21.760; p<0.001 |
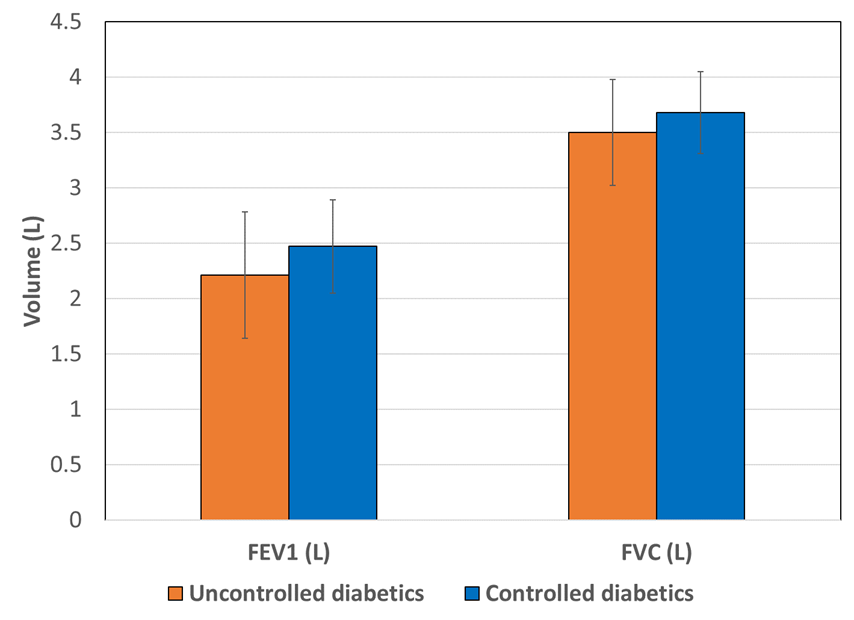
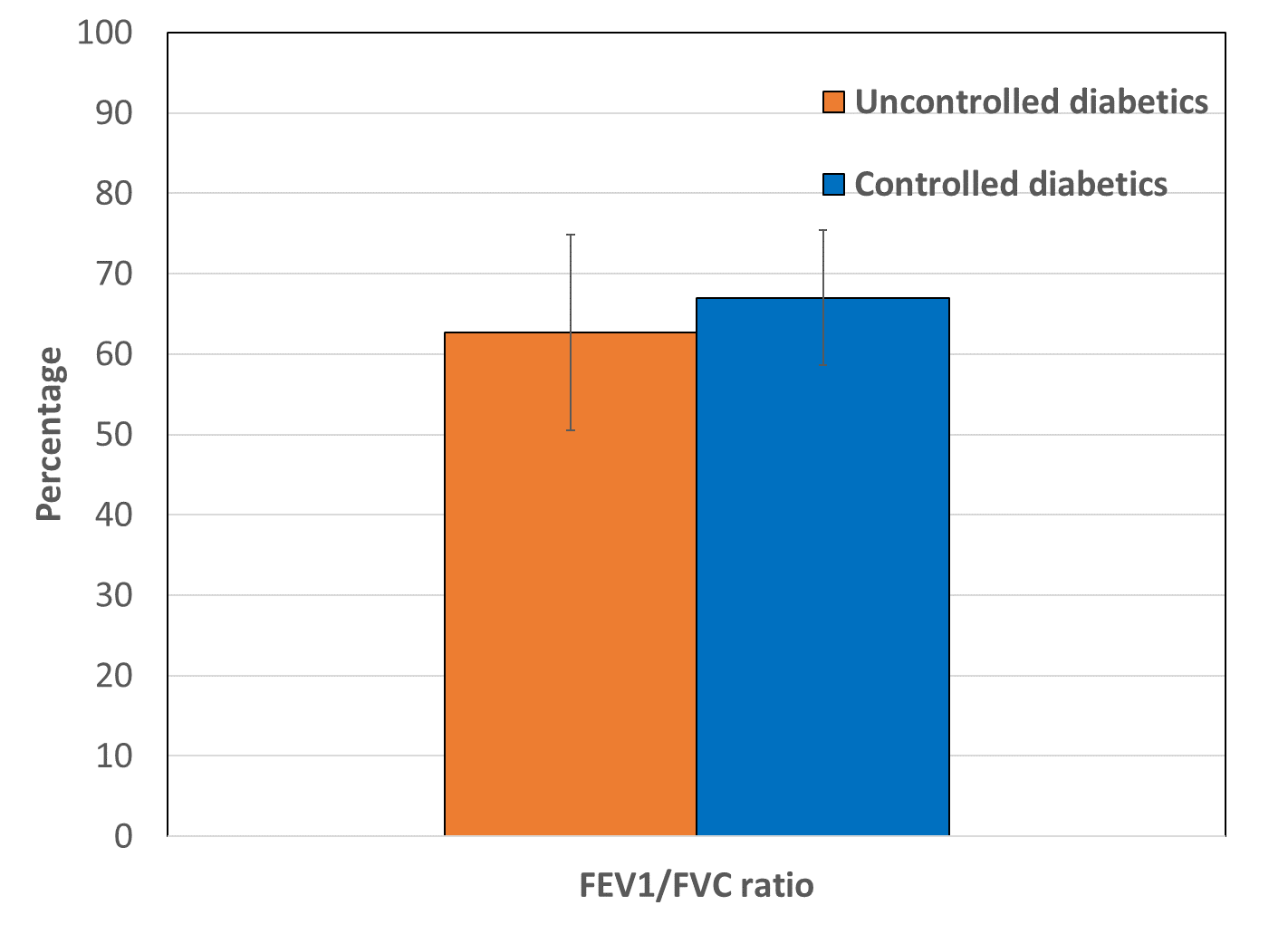
Significant decrease was found in pulmonary functions tests; Forced Expiratory Volume in 1 Second (FEV1), Forced Vital Capacity (FVC) and FEV1/FVC ratio in uncontrolled diabetics as compared to controlled diabetics (Table 1). Statistically significant (p < 0.001) decreases in FEV1 (2.21±0.57 L), FVC (3.50±0.48 L), and FEV1/FVC ratio (62.69±12.18 %) were observed in uncontrolled diabetics compared to controlled diabetics (2.47±0.42 L, 3.68±0.37 L, and 67.01±8.43%) (Figure 1, Figure 2, Figure 3). The results indicate that T2DM patients exhibit impaired lung function. Pulmonary function parameters demonstrated evidence of both restrictive and obstructive airway patterns within the groups of uncontrolled and controlled diabetics.
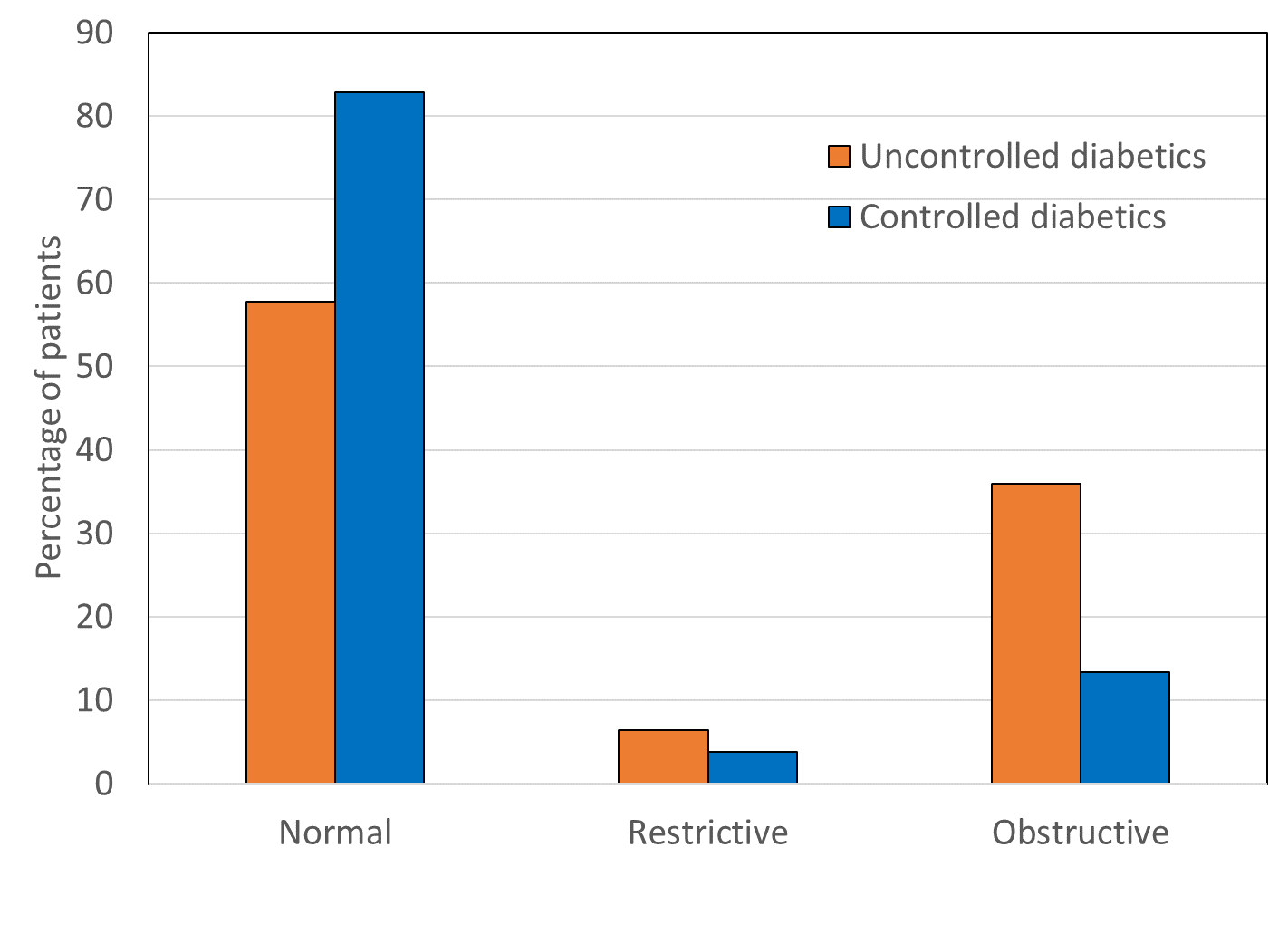
Comparison of airway pattern revealed presence of restrictive and obstructive airway in T2DM patients. Restrictive and obstructive airway abnormalities were significantly higher (c2=16.13, p<0.001) in uncontrolled diabetics (6.4%, 35.9% resp.) as compared to controlled diabetics (3.8%, 13.4% resp.).
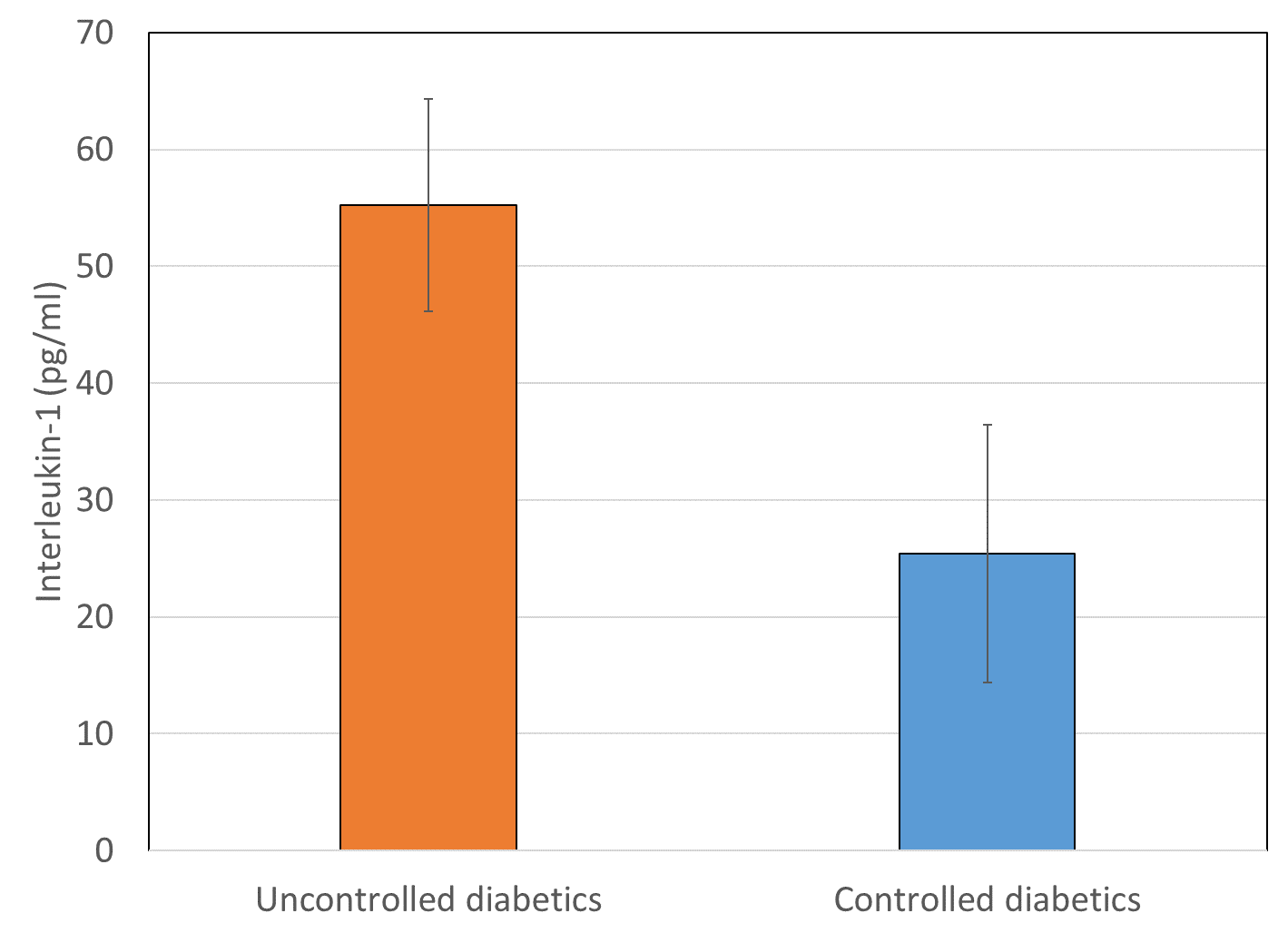
Interleukin-1β levels were found significantly increased (p < 0.001, t=21.76) in uncontrolled diabetics (55.23±9.11) as compared to controlled diabetics (25.39±11.04). Also, IL-1β levels in subjects with restrictive and obstructive airway pattern were significantly higher (p<0.001) as compared to subjects with normal airway pattern. There was no statistically significant difference between restrictive and obstructive airway pattern was observed (Figure 4).
A multivariate model was constructed to assess the independent predictors of IL-1β levels. The analysis revealed a significant association between IL-1β levels and post-bronchodilator FEV1, FVC, FEV1/FVC, and glycemic control status (r² = 0.825). In multivariate regression model, we projected IL-1β level as an independent variable with pre-bronchodilator pulmonary functions, post-bronchodilator pulmonary functions, and glycemic control status was considered as independent variable. All the three post-bronchodilator pulmonary functions (FEV1, FVC and FEV1/FVC) and glycemic control status were found to have a significant association with IL-1β levels (r2=0.825) (Table 2).
|
SN |
Variable |
β±SD |
‘t’ |
‘p’ |
|
1. |
Age (years) |
0.043±0.065 |
0.661 |
0.509 |
|
2. |
Gender (1=Male, 2=Female) |
-2.433±0.781 |
3.113 |
0.002 |
|
3. |
HbA1C (%) |
0.950±0.509 |
1.866 |
0.063 |
|
4. |
Fasting blood sugar (mg/dl) |
0.079±0.016 |
5.063 |
<0.001 |
|
5. |
Post-FEV1 (L) |
-93.54±11.30 |
8.278 |
<0.001 |
|
6. |
Post-FVC (L) |
56.53±7.90 |
7.155 |
<0.001 |
|
7. |
Post-FEV1/FVC ratio (%) |
2.56±0.37 |
6.883 |
<0.001 |
|
9. |
Group (1=Uncontrolled; 2=Controlled) |
-11.11±2.85 |
3.896 |
<0.001 |
|
10. |
Constant |
-113.10±27.54 |
4.107 |
<0.001 |
r2=0.825
Although T2DM affects more than 350 million individuals worldwide and poses a heavy socioeconomic burden, its pathogenesis is not entirely clear. The important physiological features of T2DM are insulin resistance, characterized by reduced insulin responsiveness and β-cell failure 19 . Furthermore, the COVID-19 pandemic poses an increased risk to diabetic individuals, as the infection affects the respiratory system 20 .
In this study, we assessed pulmonary function parameters in patients with T2DM. Biochemical investigations showed significantly high fasting blood glucose levels and HbA1c values in uncontrolled diabetics compared to controlled diabetics. Additionally, we observed a significant decrease in pulmonary function parameters (FEV1, FVC, and FEV1/FVC ratio) in uncontrolled diabetics compared to controlled diabetics. These results indicate impaired lung function in uncontrolled diabetics.
In recent years, the role of inflammation in the pathogenesis of T2DM has become increasingly evident. IL-1β has also been implicated in β-cell damage. Notably, β-cells release IL-1β in response to glucose stimulation. Additionally, IL-1β promotes its own synthesis within β-cells and attracts macrophages, which serve as an additional source of IL-1β and several other inflammatory mediators 21 . Increased IL-1β production have been documented in stable COPD, and these levels are further augmented during exacerbations of the disease. Elevated IL-1β levels have been found in bronchoalveolar lavage fluid and tracheal biopsy samples from both asymptomatic and symptomatic individuals with asthma 22 .
Our study findings are consistent with previously reported cross-sectional studies on T2DM patients. Klein and workers investigated the relationship between spirometric pulmonary function tests (PFTs) and diabetes complications. They reported reduced pulmonary function tests in T2DM patients as compared to predicted normal values 23 . Similarly, a research study conducted at Bijapur, evaluated pulmonary function in type 2 diabetes patients and its correlation with glycemic status and diabetes duration in an Indian population. They reported that lung function was negatively correlated with both glycemic status and diabetes duration 24 .
Another research, conducted in Nigerian T2DM population, exhibited significantly reduced pulmonary function as compared to control subjects. In this study, symptom duration, age, and BMI were considered as independent determinants of pulmonary function 25.
Haamid and co-workers observed a significant association between CRP levels and insulin resistance, obesity, and dyslipidemia. They also reported that increased TNF-α levels were strongly associated with female gender, poor glycemic control, and a strong family history of diabetes 26 .
In another study conducted by a biochemistry research team at Iran revealed a significant positive relationship between Interleukin-6 and Interleukin-1β and HbA1c, FBS, insulin, and insulin resistance. However, a significant negative correlation was found between Interleukin-6 and IL-1β and vitamin D 27 .
Studies have reported significantly elevated serum TNF-α and IL-1β in patients with diabetic retinopathy, showing a gradual increase corresponding to disease progression 28 . Smokers with COPD have also been reported to exhibit greater susceptibility to cigarette smoke. This high susceptibility leads to increased permeability, and the release of pro-inflammatory mediators, such as IL-1β and sICAM-1 29 .
The expression of IL-1β and its receptor antagonist, IL-1ra, in the normal and asthmatic bronchial wall was studied. Statistically significant increased expression of both IL-1beta and IL-1ra has been revealed in the asthmatic bronchial epithelium 30 .
Although the present study has a limitation in its relatively small sample size, the results are consistent with a number of research data discussed earlier. The results of this study offer further insight into the relationship between IL-1β levels and impaired pulmonary function in individuals with T2DM. This study explores the possibility that IL-1β serves as a pivotal cytokine in the progression of T2DM, providing essential insights into its detrimental impact on pulmonary function.
The findings of this study indicate that the simultaneous measurement of IL-1β in T2DM patients may represent a potential novel biomarker for the detection of impaired pulmonary function. The measurement of serum IL-1β levels may provide valuable information as a marker for neutrophilic inflammation, chronic airflow obstruction, and the occurrence of acute exacerbations in patients with T2DM.
Funding: None
Conflict of interest: None
Subscribe now for latest articles and news.