

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2019.v05i01.004
Year: 2019, Volume: 5, Issue: 1, Pages: 15-20
Original Article
Raksha1, Gurjeet Singh1, A D Urhekar2
1Assistant Professor, Department of Microbiology, N.C. Medical College and Hospital, Panipat, Haryana, India,
2Professor and Head, Department of Microbiology, MGM Medical College and Hospital, Navi Mumbai, Maharashtra, India
Address for correspondence:
Dr. Gurjeet Singh, Department of Microbiology, N.C. Medical College and Hospital, Panipat - 132 107, Haryana, India.
Phone: +91-8693076518. E-mail: [email protected]
Background: Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity pulmonary disease occurring in individuals with asthma or cystic fibrosis. In these patients, it is characterized by transient pulmonary infiltrates, reversible airway obstruction, eosinophilia, and evidence of hypersensitivity to the Aspergillus fumigatus. The aim of this study was standardization of antifungal drug susceptibility testing genotypic methods.
Materials and Methods: This prospective and experimental study was carried out at the Department of Microbiology and Central Research Laboratory, Mahatma Gandhi Mission Medical College and Hospital, Navi Mumbai, and gene sequencing was carried out at Eurofins Laboratory. A molecular method for standardization of drug sensitivity testing for azole drug was done using Cyp51A gene and mutation M220 and L98H. The American Type Culture Collection control strain of Aspergillus oryzae, Aspergillus niger, and Aspergillus brasiliensis was obtained from Microbiologics Inc., USA.
Results: Azole drug resistance by polymerase chain reaction (PCR) revealed 48/60 (80%), and azole drug resistance showed L98H and M220 genes in 32/60 (60%). Gene sequencing of Aspergillus species in patient samples revealed M220 and L98H mutation in cyp51A gene of Aspergillus Species.
Conclusions: Azole drug resistance by PCR showed L98H and M220 in cyp51A gene. Gene sequencing showed similar resistance by PCR.
KEY WORDS:Azole, polymerase chain reaction, gene sequencing, cyp51A.
Allergic aspergillosis is a hypersensitivity disease manifesting in several clinical forms, including allergic susceptible bronchopulmonary aspergillosis allergic bronchopulmonary aspergillosis (ABPA). ABPA is described by bronchiectasis, wheezing, pulmonary infiltrates, and sputum containing brown plugs.[1] Asthmatic and cystic fibrosis patients are primarily at risk.[2] As ABPA is a long-term condition, its prevalence is higher than invasive aspergillosis (IA), but its annual incidence or rate of new diagnoses is probably lower. In addition to the conventional corticosteroid treatment, itraconazole (ITC) antifungal therapy has been shown to be advantageous in 60% of cases.[3]
Aspergillus bronchitis (or aspergillary bronchitis, as it was first called)[4] may occur in patients with underlying pulmonary or airway pathology,[5] especially in the context of lung transplantation.[6,7] Antifungal therapy is probably effective if it has not evolved into pseudomembranous Aspergillus tracheobronchitis, which is usually fatal.
The azoles are the largest and most widely used class of antifungal drugs. Although voriconazole (VRC) is regarded as first-line therapy for IA,[5] ITC is still commonly used for chronic non-invasive forms of aspergillosis. Resistance to ITC in Aspergillus fumigatus, the species which causes the vast majority of cases of allergic aspergillosis is well recognized. In our experience, it occurs mainly in patients with chronic forms of aspergillosis, particularly chronic cavitary pulmonary aspergillosis with aspergillomas. The frequency of ITC resistance in clinical A. fumigates strains since the turn of the millennium (when most cases have been reported) is between 2% and 3%, and it can increase up to 6% depending on the area from which it is reported.[8-10] Cross-resistance between other azole drugs has also been reported.[11,12]
Intrusive aspergillosis IA has risen as a perilous contamination in immunocompromised patients. An expansion in contaminations due to azole-safe A. fumigatus has been watched lately,[13-15] prompting a higher case casualty rate among patients with azole-safe obtrusive aspergillosis.[16] The rule obtained or watched segment giving azole sedate obstacle entangles point changes in the 14-sterol demethylase quality (cyp51A) just as extended cyp51A verbalization due to a couple repeat (TR) sponsor modification.[5,17-20] As yet, the recognizable proof of assurance from azoles (itraconazole, voriconazole, posaconazole) is totally established on positive social orders from clinical isolates, and the to this point existing PCR measures to recognize both the azole restriction mediating pair repeat and the single nucleotide polymorphisms were performed with culture tests and depend upon positive culture disclosures from clinical segregates.[17,18,21-23] Up to now, just a single PCR-based measure that distinguishes cyp51A transformations straightforwardly in clinical examples (sputum) from patients with pneumonic ailments such as hypersensitive bronchopulmonary aspergillosis ABPA and ceaseless aspiratory aspergillosis has been depicted. In this investigation, tests from a couple of patients experiencing obtrusive pneumonic aspergillosis invasive pulmonary aspergillosis were additionally tried, yet these examples did not yield results due to a deficient measure of test remaining.[24] From a clinical perspective, in any event in patients with hematological malignancies at high hazard for IA, apparent Aspergillus culture yields are rare and a culture-based determination of IA is once in a while achieved.[25,26]
This prospective, molecular, and experimental study was conducted at Microbiology Laboratory and Central Research Laboratory, Mahatma Gandhi Mission Medical College and Hospital, Navi Mumbai, Maharashtra, India. Gene sequencing was done at Eurofins Laboratory, Bengaluru, with Head Office in the UK.
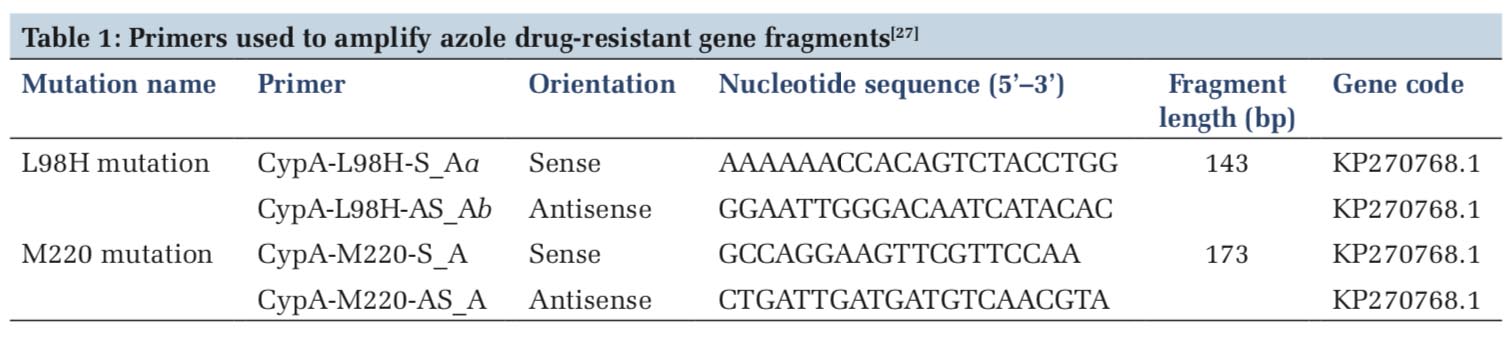
Primer sequences
The oligonucleotide primers used in this study are described in Table 1. The primers were obtained from Sigma, USA.
PCR specification
PCR amplifications were performed in accordance to a procedure as followed by Spiess et al.[27] According to the procedure Master Mix “GoTaq Green Master Mix” (Promega Bio Sciences, LLC., USA), 5 μl DNA, 20 pmol of primers were added and mixed to obtain 50 μl final volume of the PCR mix.
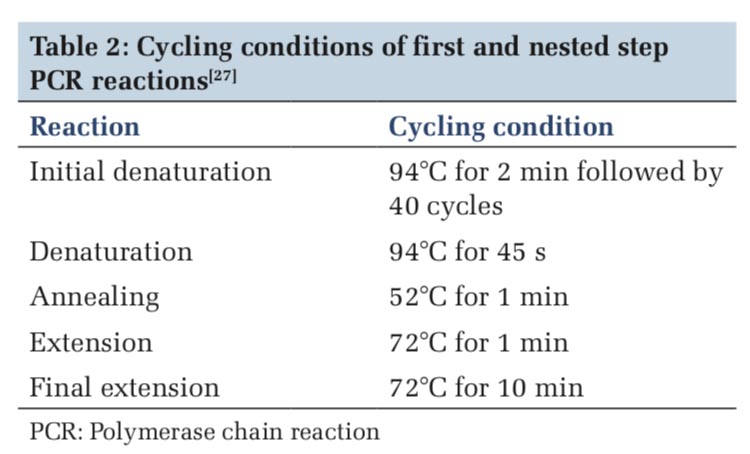
Reaction conditions
Cycling conditions of first and nested-step PCR reactions was performed according to previously published work.[27] The reaction and the cycling condition in PCR were followed step by step, i.e. firstly initial denaturation of the DNA occurred at 94°C for 2 min, followed by 40 cycles, then again denaturation occurs at 94°C for 45 s, annealing at 52°C for 1 min then extension at 72°C for 1 min and final extension at 72°C for 10 min (Table 2).
Agarose gel electrophoresis:
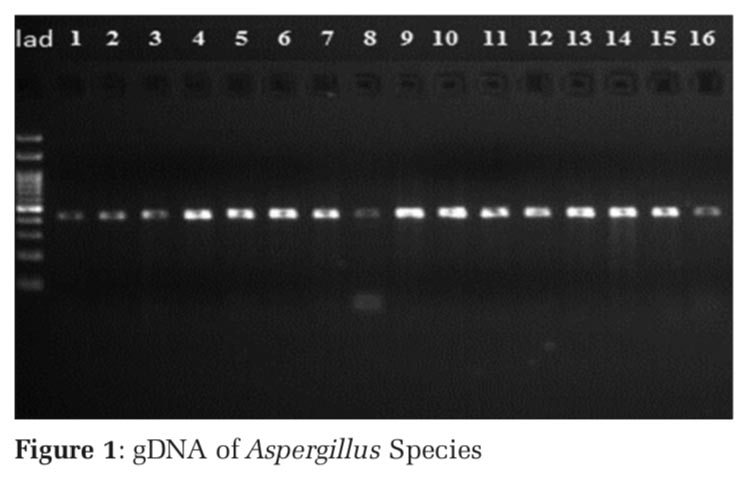
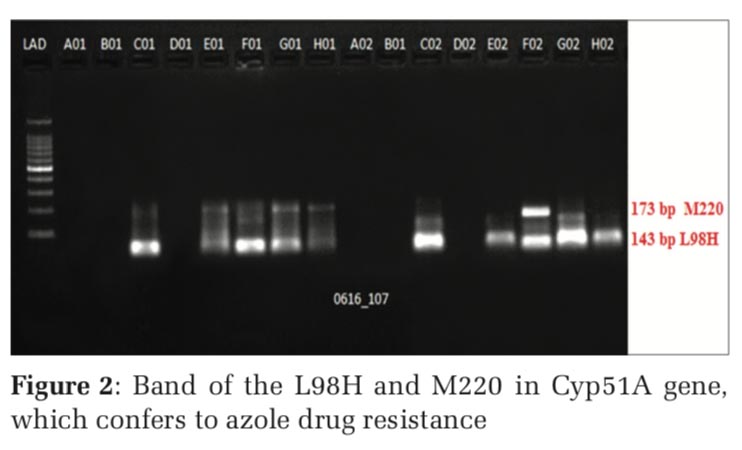
The PCR products along with the appropriate ladder (Bioline, India) were subjected to electrophoresis in a 1.5% agarose gel using 1 X Tris Acetate EDTA (TAE) buffer. The presence of band of the L98H and M220 in Cyp51A gene in gel electrophoresis confers to Azole drug resistance (Figures 1 and 2).
Gene sequencing
Gene sequencing of amplified product was done using Sanger method (BigDye Terminator chemistry, gel: Pop 7 polymer) 3730XL DNA Analyzer (Applied Biosystems®).
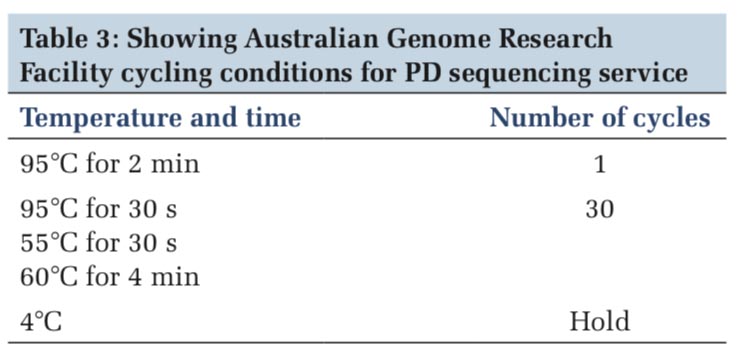
Cycle sequencing
AustralianGenomeResearchFacility(AGRF)cycling conditions for PD sequencing service was performed according to the Table 3. The temperature and time and the number of cycles condition were followed step by step, i.e. at 95°C for 2 min and the number of cycle was one, at 95°C for 30 s and 55°C for 30 s, the number of cycle were 30 for each and hold at 4°C (Table 3).
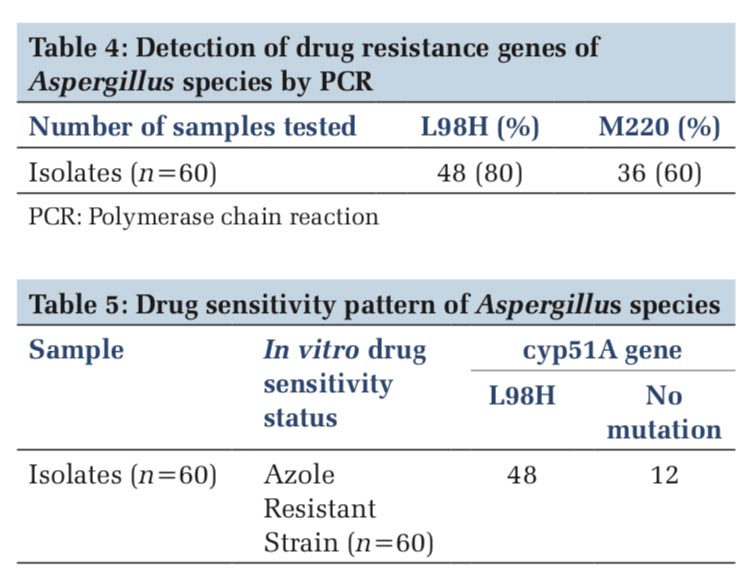
The present examination was directed for recognizing azole tranquilizes safe transformations in Aspergillus species. A total of 60 isolates were included in this study out of that 80% was L98H gene and 60% M220 genes (Table 4).
Detection of L98H and M220 mutations in cyp51A gene of Aspergillus species was done by polymerase chain reaction (PCR).
Sequence analysis of L98H and M220 in cyp51A gene
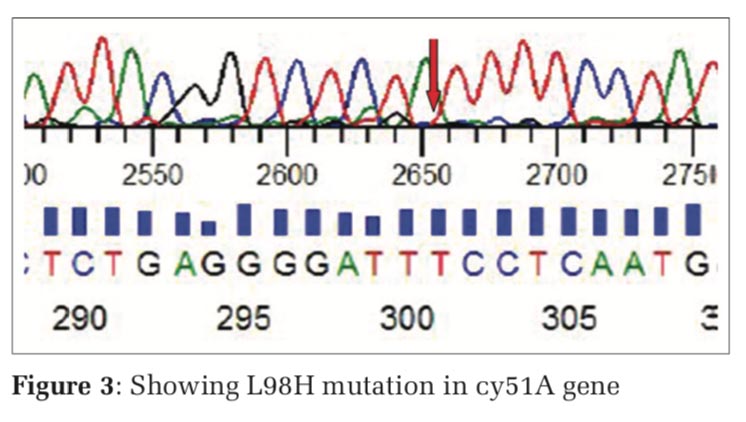
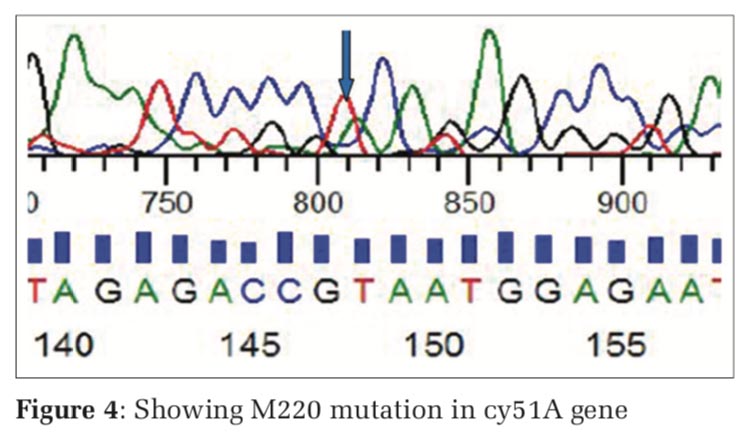
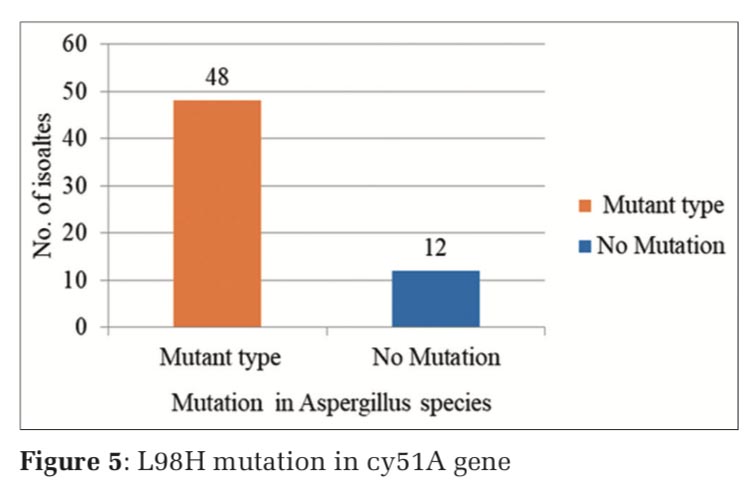
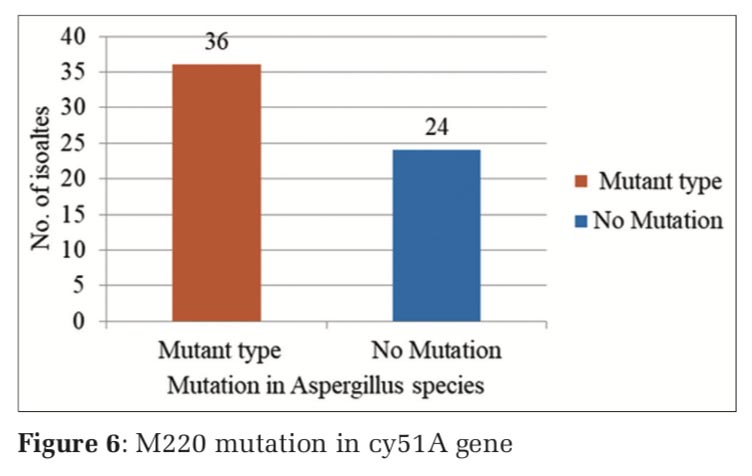
The nested PCR products of L98H and M220 in cyp51A gene from the above samples were directly sequenced in both directions, using an 3730XL DNA sequence (Sanger method, BigDye Terminator chemistry and Pop 7 polymer gel) from Eurofins IT Solutions India Pvt. Ltd. (Bengaluru, India). Total 60 isolates which were resistant to azole drug were further confirmed by gene sequencing analysing using Sanger method, out of which 48 isolates were confirmed with L98H mutation whereas 12 isolates showed no L98H mutation (Table 5, and Figure 3) and 36 isolates were confirmed with M220 genes however 24 isolates showed no M220 mutation (Table 6, and Figure 4).
In this chapter, drug resistance pattern of azole was studied in Aspergillus isolates by PCR and azole drug resistance mutations were studied by gene sequencing.
Cyp51A–L98H-S Aa/Ab and Cyp51A-M220-SA/ AS-A primers were used in the study. The study was done on 60 Aspergillus isolates. About 48/60 (80%) Aspergillus isolates showed the presence of L98H, whereas 32/60(60%) showed M220 azole drug resistance mutant genes.
In vitro drug sensitivity of 60 Aspergillus isolates revealed 48 azole resistance strains and 12 azole- sensitive strains. All 48 azole drug resistance strains showed Cyp51A (L98H) mutation, whereas 12 strains did not show mutation and drug resistance.
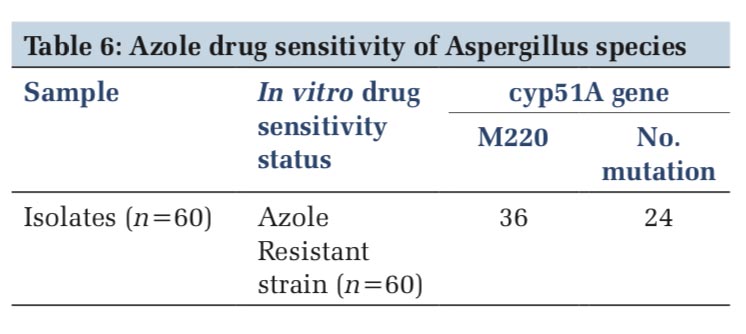
Thirty-two azole resistance strains showed M220 mutation, where in 28 strains, no mutation was observed.
Gene sequencing revealed (TTC→TTT) mutation in L98H mutant genes and (CGC→CGT) in M220 mutant genes (Figures 5 and 6).
Our results correlate with Bueid et al.[13] who reported that 78% of cases have multi-azole drug resistance.
In assurance of the opposition system in Cyp51A quality toward ITC, succession examination and level Cyp51A quality articulation were contemplated. Preliminary sets were chosen for Cyp51A quality enhancement dependent on past examinations. In light of arrangement examination, A. fumigatus disengages C21, C53, M310, M2470, and UZ291 demonstrated the nearness of L98H transformation. In the meantime, M220 change was distinguished in UZ165 confine, and both L98H and M220 transformations were identified in UZ23 and UZ685 disengages. The pair rehash TR change was resolved dependent on the amplicon estimate (100 bp), and this transformation was not recognized as there was no expansion of 34 bp in the amplicons.[12]
In light of past research,[13] the cross-obstruction toward azole drugs is identified with articulation dimension of Cyp51A quality which is because of a Tandem Repeat 34 bp in the advertiser locale and amino corrosive changes at area 98 leucine (TRL98H). The L98H, M220, and Tandem Repeat were not recognized at all in Aspergillus niger confines, and the opposition in A. niger might be due to different elements.[6] In the meantime, the opposition segregates which do not demonstrate any quality articulation or L98H, M220, and TR change could be due to the essence of different transformations, for example, F46Y, G89G, M172V, N248 T, D255E, L358 L, E427K, C454C, L358 L, or efflux siphon component.[14]
While the dimension of Cyp51A quality articulation is higher particularly in safe A. niger segregates, this expansion is not huge. The G427S transformation was recognized in M046, M407 and M1772 separates of A. niger which may show a critical connection with Itraconazole antifungal medication opposition. Anyway it was unrealistic to enhance alternate disconnects with the preliminary pair utilized. This is on the grounds that A. niger is an animal groups complex comprising of various distinctive strains[12] and a few other preliminary sets would need to be utilized so as to enhance alternate disconnects.[28,29]
In our study, we found that molecular method is time-effective method giving results within short time required for the test and identification of azole drug resistance, which help doctor to regulate proper antifungal drugs and minimize morbidity and mortality of patients suffering from IA. Mutations such as L98H and M220 in gene cyp51A are very helpful biomarkers for the identification of azole medicate obstruction in aspergillosis patients.
Subscribe now for latest articles and news.