

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.v11.i3.24.259
Year: 2025, Volume: 11, Issue: 3, Pages: 275-281
Original Article
Ambresh S Badad1 , R N Ankitha2 , Shruti A Badad3
1Professor, Department of Dermatology, Venerology and Leprosy, Mahadevappa Rampure Medical College, Kalaburagi, Karnataka, India,
2Post Graduate Resident, Department of Dermatology, Venerology and Leprosy, Mahadevappa Rampure Medical College, Kalaburagi, Karnataka, India,
3Assistant Professor, Department of Ophthalmology, Gulbarga Institute of Medical Sciences, Kalaburagi, Karnataka, India
Address for correspondence:
Ambresh S Badad, Professor, Department of Dermatology, Venerology and Leprosy, Mahadevappa Rampure Medical College, Kalaburagi, Karnataka, India.
E-mail: [email protected]
Received Date:09 August 2024, Accepted Date:23 May 2025, Published Date:14 November 2025
Background: Pemphigus vulgaris (PV) is an autoimmune blistering disease caused by autoantibodies, primarily IgG that target desmoglein-3 and sometimes desmoglein-1. Traditionally, mainstay of treatment is systemic corticosteroids in combination with immunosuppressants. Rituximab is a novel monoclonal antibody used in pemphigus vulgaris to target B cells, effectively reducing autoantibody production and controlling disease activity.Top of FormBottom of Form Objective: We aimed to assess the efficacy of low dose intravenous and intralesional rituximab therapy in treating oral pemphigus vulgaris subsequently to evaluate durability and safety profile of treatment. Materials and methods: 50 patients of both sexes falling in age group of 18 and 60 with oral pemphigus vulgaris were included in the study. Patients were administered IV Rituximab (500mg/50ml) along with intralesional injection at the site of oral lesions. Response to treatment was evaluated based on remission of lesions and monitoring of adverse reactions which were carried out biweekly in the first two months followed by monthly assessment for a period of 6 months. Results: The results were statistically analysed using Chi square test and statistical significance (p value) methods. An excellent result was obtained in 72% (36/50) where patients achieved complete remission within 3 months, defined as absence of new lesions and healing of existing ones. 16% (8/50) showed good response, experienced mild relapses at 6 months which was successfully managed with topical steroids. Conclusion: Low dose rituximab therapy along with intralesional injection provides excellent results with significant reduction in adverse effects & cost associated with high dose rituximab therapy.
Keywords: Low dose rituximab, Intralesional, Pemphigus Vulgaris
Pemphigus vulgaris (PV) is a rare and potentially life-threatening autoimmune disorder that primarily affects the skin and mucous membranes. It is characterized by the formation of painful blisters and erosions, with the oral cavity being one of the most commonly affected sites. 1 The disease has a significant impact on patient’s quality of life, causing difficulty in eating, speaking, and maintaining oral hygiene. Despite advancements in treatment options, managing oral PV remains a challenge due to the chronic nature of the disease, potential for serious complications, and the side effects associated with traditional therapies.
In recent years, rituximab, a chimeric monoclonal antibody that targets CD20 on B cells, has emerged as a promising treatment for PV. 2 By selectively depleting B cells, which are responsible for producing the autoantibodies that cause the disease, rituximab offers a more targeted approach compared to conventional immunosuppressants. 3 However, the standard intravenous (IV) rituximab regimens require high doses, which can lead to an increased risk of adverse events such as infections, infusion reactions, and long-term immunosuppression. 4 Additionally, the high cost of rituximab poses a significant economic burden, limiting its accessibility in many healthcare settings.
This study introduces a novel treatment protocol that combines low-dose IV rituximab with intralesional rituximab injections directly into oral lesions. 5 By reducing the systemic dose and targeting the affected areas directly, we aimed to enhance the therapeutic efficacy while minimizing systemic side effects and treatment costs. This approach represents a potential paradigm shift in the management of oral PV, addressing key limitations of current therapies and potentially improving patient outcomes. 6
A prospective, interventional study was conducted at a tertiary care dermatology centre in 50 patients of oral pemphigus vulgaris. The study duration was of 7 months comprising of one month of treatment phase and a six months follow-up period.
50 patients falling in the age group of 18-60 years with histologically and immunologically confirmed oral pemphigus vulgaris who meet at least one of the following:
Resistant to conventional therapy (high-dose corticosteroids ± immunosuppressants) for at least 3 months.
Contraindicated for standard immunosuppressants due to comorbidities.
Experiencing a relapse despite high-dose corticosteroids (≥1 mg/kg/day prednisone).
Pregnant and lactating women.
Children under the age group of 18 years.
Type 1 hypersensitivity or history of anaphylaxis to murine proteins, CHO cells or to other components of Rituximab.
Active Hepatitis B infection, Tuberculosis.
History of or current malignancy.
Patients were enrolled based on inclusion and exclusion criteria after obtaining written and informed consent. Pre-treatment assessments were conducted including a detailed review of the patient's history with pemphigus vulgaris, prior treatments and any accompanying medical conditions. A comprehensive clinical examination was also performed to evaluate the extent and severity of oral lesions. Complete hemogram, erythrocyte sedimentation rate, renal function tests, and liver function tests, serology for HIV infection, hepatitis B surface antigen, anti-HCV (hepatitis C virus) antibody, and anti-HBc (hepatitis B core) antibody, Mantoux test, chest X-ray, Cardiology evaluation, electrocardiography and echocardiogram (if advised by a cardiologist), Estimation of immunoglobulin (Ig) G level in serum, Baseline serum level of antidesmoglein 1 and 3 antibodies, Ultrasound abdomen (if indicated clinically based on symptoms and clinical examination) were conducted in the initial visit. Premedication with Tab Paracetamol 650 mg, Inj. Hydrocortisone 100 mg IV, Inj Pheniramine maleate 2 cc IV were given.
IV rituximab was administered at a dose of 500 mg in 500 ml NS with first dose of rituximab infusion starting at 50 ml/hr. 50 mg increments were made in infusion rate every 30 mins to a maximum of 400 mg/hr. Initially, the infusion was started at a rate of 30 ml per hour for the first 30 minutes, delivering a volume of 15 ml. This cautious beginning allows for monitoring of any immediate adverse reactions. Subsequently, the infusion rate was increased to 60 ml per hour for the following 30 minutes, delivering 30 ml of Rituximab. As the treatment progresses, the infusion rate was further escalated to 90 ml per hour until the total volume of 455 ml was infused over a period of approximately 5 hours. The entire infusion process for the first dose typically spans a total duration of 6 hours, ensuring the medication is administered safely and effectively while minimizing potential complications.
Patients were given 10 mg rituximab per session under local anesthesia (2% lidocaine) with dosages modified according to the severity of the lesion. Injections are targeted directly at the base and margins of oral lesions using a 30-gauge needle, employing multiple angles to surround the lesion effectively within the same plane.
The patient's vital signs including pulse rate, blood pressure, respiratory rate and pulse oximetry were monitored at 30-minute intervals during the administration of treatment. Symptoms such as fever, rigors, nausea, vomiting, headache, hypotension, and abdominal pain were closely observed for signs of infusion reactions. Emergency medications including epinephrine, hydrocortisone, chlorpheniramine maleate, and furosemide were prepared in appropriate doses as a precautionary measure to manage potential adverse reactions. In the event of serious adverse effects like angioedema or anaphylaxis, the infusion was immediately halted and the patient received prompt treatment tailored to the specific symptoms. Following such incidents, efforts were made to investigate and treat the underlying cause responsible for the reaction, ensuring comprehensive patient care and safety throughout the treatment process.
Throughout the treatment course for pemphigus vulgaris, patients were scheduled for visits initially every 15 days over 2 months, followed by monthly visits for the subsequent 6 months. Each visit included a thorough assessment and monitoring protocol aimed at ensuring effective disease management and patient well-being. During these visits, a thorough clinical assessment of oral lesions & documentation of lesion progression through photography for objective comparison was conducted. Patients were also closely monitored for infections, alongside performing regular complete blood count, liver function tests, and renal function tests to evaluate overall health and medication tolerance. At 3-month and 6-month intervals, additional assessments included measuring serum IgG levels and anti-desmoglein antibody titers to track treatment response and utilizing the Autoimmune Bullous Disease Quality of Life (ABQOL) questionnaire to assess the impact of the disease and treatment on the patient's quality of life. This comprehensive approach ensured continuous evaluation, adjustment of treatment strategies as needed, and comprehensive support for patients throughout their treatment journey.
The results were statistically analysed using Chi square test and statistical significance (p value) methods.
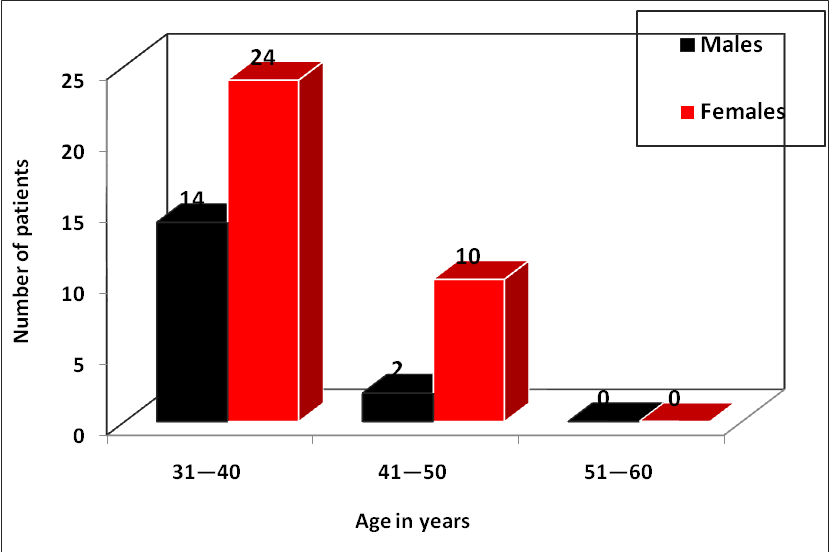
The study observed that the majority of oral pemphigus patients (38, 76.0%) belonged to the age group of 31–40 years, followed by 12 patients (24.0%) in the age group of 41–50 years. Oral pemphigus showed a higher prevalence in the age range of 31–40 years. The minimum age of patient was 31 years, and the maximum age was 47 years. The mean age of male patients was 36.87 years, and the mean age of female patients was 38.09 years. The mean age of all patients was 37.70 years. There was no statistically significant difference in age between genders (P > 0.05).
Also, the study observed that out of 50 oral pemphigus patients sampled, 45 (90.0%) had oral involvement, 4 (8.0%) showed minimal involvement, and 1 (2.0%) patient showed no involvement. Investigations were conducted on all 50 patients (100.0%).
|
Treatment Response |
Response after baseline visit (1 st dose) |
Response after 15th day (2 nd dose) |
3rd month follow up |
|||
|
No |
% |
No |
% |
No |
% |
|
|
Excellent response |
30 |
60.0 |
36 |
70.0 |
42 |
76.0 |
|
Good response |
14 |
28.0 |
10 |
22.0 |
4 |
18.0 |
|
No significant response |
6 |
12.0 |
4 |
8.0 |
4 |
8.0 |
|
Total |
50 |
100.0 |
50 |
100.0 |
50 |
100.0 |
|
Base line v/s after 15 days |
χ2= 1.612, P = 0.446, Not significant |
|||||
|
Base line v/s 3 months |
χ2= 7.955, P = 0.018, Significant |
|||||
|
After 15 days v/s 3 months |
χ2= 3.032, P = 0.219, Not significant |
|||||
Comparison of treatment response:
After the 15th day (2nd dose), 6 patients showed excellent response, which increased from those initially categorized as good responders, and 2 patients previously showing no significant response progressed to good response. However, these changes were not statistically significant (P > 0.05).
At the 3rd-month follow-up, 42 patients exhibited excellent response, 4 patients showed good response, and 4 patients showed no significant response. Comparing this to the baseline response, there was a statistically significant change in treatment response (P < 0.05).
At the 3rd-month follow-up, 42 patients showed excellent response, 4 patients showed good response, and 4 patients showed no significant response.

In evaluating treatment outcomes for pemphigus vulgaris, primary goals were to achieve complete remission (CR), characterized by the absence of new lesions and substantial healing of existing one. Partial remission (PR) was defined as a ≥50% reduction in lesions. Secondary endpoints focused on additional measures such as assessing the time required to achieve CR or PR, monitoring the 6-month relapse rate to gauge the necessity for increased therapy due to new lesions. These comprehensive endpoints collectively provided a thorough assessment of treatment effectiveness, safety, and patient outcomes in managing pemphigus vulgaris.
In the current study conducted on 50 patients with oral pemphigus vulgaris, the majority were females, accounting for 76%, while males constituted 24% (Figure 1). The highest number of patients fell into the 30-45 age group. The resolution of body lesions preceded the improvement of oral lesions in majority of patients. Among the participants, 36 individuals achieved an excellent response to treatment, characterized by the absence of new lesions and healing of existing ones. A total of 16% (8 out of 50) showed a good response, experiencing mild relapses at 6 months that were effectively managed with topical steroids. However, 12% (6 out of 50) showed no significant response to therapy (Figure 2).
Throughout the follow-up visits, there was a significant decline in serum levels of anti-desmoglein 1 and 3 antibodies indicating substantial improvement in disease activity. The Autoimmune Bullous Disease Quality of Life (ABQOL) questionnaire underscored considerable enhancements in patient quality of life attributable to disease management and treatment.
Complications that had been reported with rituximab therapy included infusion reactions, infections, hypogammaglobulinemia, tumor lysis syndrome, progressive multifocal encephalopathy, and Hepatitis B reactivation. However, in our study involving low-dose intravenous and intralesional rituximab therapy, we encountered only 3 incidents (6%) where patients complained of chills following the initiation of infusion. These reactions were promptly managed, and therapy continued without interruption.
Despite the promising outcomes, certain limitations of this study should be acknowledged. Our sample size of 50 patients, while providing valuable insights, may not be sufficient to detect rare adverse events or establish definitive long-term safety profiles. A significant limitation of rituximab therapy remains it’s high cost, which poses a substantial financial burden for many patients. Despite implementing a low-dose protocol to minimize expenses, the cost of treatment remains a significant barrier impacting both access to care and patient compliance.
The single-center nature of our study limited the diversity of patient demographics and socioeconomic backgrounds, which is particularly relevant when evaluating treatment affordability across different healthcare settings. A broader, more diverse patient population would provide more generalizable results regarding both efficacy and economic feasibility of this novel protocol.
Pemphigus is a rare autoimmune disease characterized by the formation of blisters and erosions on the skin and mucous membranes.7 Management of pemphigus remains challenging as it requires a careful balance between controlling the disease and managing treatment-related side effects such as infections and long-term complications of immunosuppressive therapy.
Traditionally, treatment for pemphigus has centered around immunosuppressive therapies. Corticosteroids are typically the first-line treatment followed by immunosuppressive drugs like azathioprine, mycophenolate mofetil and methotrexate which are often used in combination with corticosteroids to achieve better disease control. 8
In recent years, Rituximab, a monoclonal antibody targeting B cells has emerged as a promising treatment option for pemphigus. It works by depleting B cells responsible for producing antibodies that attack desmogleins, thereby helping to control the autoimmune process more effectively than traditional therapies in some cases. 9
In the current study, 50 patients of both genders, aged 18 to 60 years and diagnosed with oral pemphigus vulgaris, were treated with intravenous Rituximab (500mg/50ml) in addition to intralesional injections (Figure 3, Figure 4 : Patient 1, 2- fig C) directed at the oral lesion sites. Among the participants, 36 individuals demonstrated an excellent response to treatment marked by the absence of new lesions and the healing of existing ones. (Figure 3 : Patient 1-fig A, fig B) A total of 16% (8 out of 50) exhibited a good response, with mild relapses observed at 6 months that were effectively controlled using topical steroids. (Figure 4 : Patient 2-figure A, B) However, 12% (6 out of 50) showed no significant response to therapy.
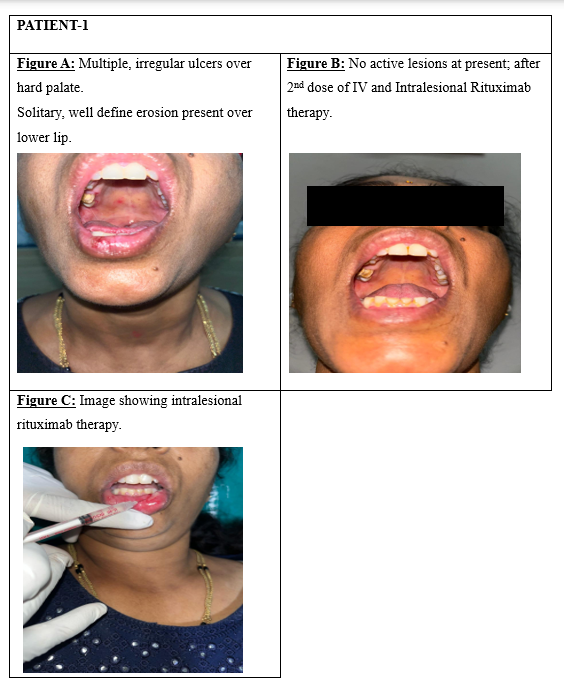
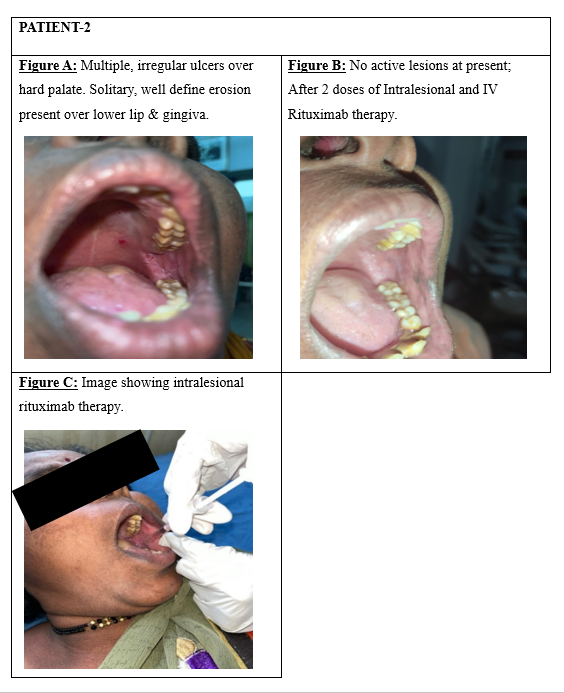
Horváth et al. demonstrated that low-dose IV rituximab (500 mg) can be effective in PV, suggesting that the standard 1000 mg dose may be higher than necessary which is in accordance with our study thereby reducing systemic adverse effects associated with high dose regimen. 6
Vinay et al. demonstrated efficacy with intralesional rituximab injections for challenging oral PV lesions, emphasizing the promise of targeted therapy. This approach was explored in our study, affirming enhanced local efficacy by directly addressing oral lesions through intralesional injections. 8
Colliou et al. had found that rituximab induced prolonged depletion of desmoglein-specific B cells, suggesting that its effects could be durable even with lower doses. This finding aligned with our study, which also provided insights into the durability of response and long-term safety of this targeted approach. 9
This methodology was designed to rigorously evaluate the efficacy, safety, and cost-effectiveness of the novel low-dose IV and intralesional rituximab protocol (Dr Badad’s protocol) for oral PV. By combining systemic and targeted therapy, the goal was to achieve optimal disease control while minimizing the risks and costs associated with standard rituximab regimens. The comprehensive follow-up and outcome measures aimed to provide valuable insights into the durability of response and long-term safety, addressing key knowledge gaps in PV management.
Low-dose intralesional rituximab therapy has shown effectiveness similar to conventional dosing methods with notable reductions in adverse effects and treatment expenses. Additionally, this approach offered valuable insights into the long-term safety and effectiveness of rituximab therapy for Pemphigus vulgaris particularly in the context of Oral Pemphigus. This research has the potential to guide clinical decisions and enhance treatment approaches for PV patients ultimately enhancing their quality of life.
Nil.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Nil.
There are no conflicts of interest.
Subscribe now for latest articles and news.