

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2019.v05i01.003
Year: 2019, Volume: 5, Issue: 1, Pages: 12-14
Original Article
Cengiz Kadıyoran
Department of Radiology, Necmettin Erbakan University, Meram Faculty of Medicine, Meram/ Konya, Turkey
Address for correspondence:
Prof. Cengiz Kadıyoran, Assistant Professor, Department of Radiology, Necmettin Erbakan University, Meram Faculty of Medicine, Meram/ Konya - 42080, Turkey. Phone: 90 332 2216337. E-mail: [email protected]
Purpose: The etiology of adenomyosis is not well understood, but adenomyosis is a common disease. In our study, we aimed to investigate the relationship between adenomyosis and subcutaneous fat tissue thickness by magnetic resonance imaging (MRI).
Materials and Methods: Eighty patients were included in the study and classified into two groups according to their MRI diagnosis. The first group includes patients diagnosed adenomyosis with MRI and the control group consisted of patients who had undergone pelvic MRI for any reason not diagnosed with adenomyosis. The thickness of the subcutaneous fatty tissue was measured at the localization of the first sacral vertebral corpus in both patient groups. In addition, the number of births, cesarean section, and additional pelvic pathologies were compared.
Results: Subcutaneous fatty tissue thickness was significantly increased in patients with adenomyosis compared with the control group (P < 0.0005). There was a significant difference in the number of three and more pregnancies in the adenomyosis group (n=23) compared to the control group (n=7). No significant difference was found between the two groups of patients in the history of cesarean section.
Conclusions: Adenomyosis is a disease of unknown etiology. Obesity and consequently an increase in thickness in the subcutaneous fatty tissue is one of the important risk factors for the development of adenomyosis.
KEY WORDS:Adenomyosis, subcutaneous fat thickness, obesity, magnetic resonance imaging.
Adenomyosis is a common disease, but its etiology is not fully understood.[1,2] Dysmenorrhea, menorrhagia, andchronicpelvicpainarecommonsymptoms.[3,4]It affects women of all age groups. Endometrial gland and stroma extend into the myometrium, and diagnosis is made after histopathological examination.[5-7] In magnetic resonance imaging (MRI), the junctional zone thickness of 12 mm and above is diagnostic foradenomyosis.Theetiologyincludesmultiparity, previous uterine surgery (history of cesarean section), and middle age.[4,8] In the present study, we investigated the relationship between adenomyosis and subcutaneous fatty tissue thickness in the sacral region in patients diagnosed with adenomyosis on MRI. There are not enough studies in the literature.
To investigate the relationship between adenomyosis and subcutaneous fatty tissue thickness, a Picture Archiving and Communication System (PACS) archive was screened and 40 patients who were diagnosed with adenomyosis on MRI were included in the study. In this group of patients, junctional zone thicknesses of 12 mm and above were determined as the criteria of adenomyosis by sagittal T2-weighted MRI images. The control group consisted of 40 patients who underwent pelvic MRI scans for any reason by scanning the PACS archive. The control group consisted of patients with junctional zone thickness below 12 mm in sagittal T2-weighted MRI examination. Both the patient and the control group, subcutaneous fatty tissue thickness measurement was performed at the level of the middle segment of the corpus of first sacral (S1) vertebra with the aim of providing the standard point. T2-weighted sagittal images were used for measurement in both patient groups, and skin thickness was not included in the measurement. Patients with focal adenomyosis, partial hysterectomy, oophorectomy, spinal surgery for lumbosacral region, and pelvic radiotherapy were not included in the study.
In both the groups, the number of births of the patients, the history of cesarean surgery, and accompanying gynecological pathologies were noted. After the diagnosis of adenomyosis, the pathology results of the patients who underwent hysterectomy were compared with the imaging findings. The available data were compared using the t-test.
In our study, we investigated the relationship between adenomyosis and subcutaneous fatty tissue thickness, one of the findings on obesity; The mean age of the patients with the diagnosis of adenomyosis was 43.5 ± 5.5 years, and the mean age of the control group was 43.6 ± 4.5 years. Subcutaneous fatty tissue thickness at S1 level was significantly increased in patients with adenomyosis (Figure 1) compared to patients without adenomyosis (Figure 2) (P < 0.0005).
Twenty-three (57.5%) patients with the diagnosis of adenomyosis and 7 (17.5%) patients in the other group had three or more pregnancy history. The difference between the two groups was significant. When the two groups were compared with cesarean section history, eight patients in the adenomyosis group and seven patients in the control group had cesarean section history. There was no statistically significant difference between cesarean section history of each group. The most common pelvic pathology in both the groups was nabothian cysts, and no significant difference was found between the two groups in terms of the incidence of nabothian cysts. When leiomyoma presence was compared in both the groups, leiomyoma was detected in 6 patients in the adenomyosis group and leiomyoma was detected in 27 patients in the control group. The difference was statistically significant.
Five of 40 patients in adenomyosis group underwent surgical hysterectomy, and the diagnosis of adenomyosis was confirmed histopathologically.
We found that adenomyosis is related to subcutaneous fatty tissue thickness, which is one of the indirect findings on obesity and this result is similar to the study of Trabert et al.[9] that adenomyosis and obesity connection are mentioned. Trabert et al.[9] compared normal and overweight women in their study and found that the risk of adenomyosis in overweight/obese patients increased compared to normal weight patients and there was a strong link between obesity and adenomyosis.
Similarly, Koike et al.[10] and Templeman et al.[11] emphasized that obesity plays a role in the etiology of adenomyosis. Ferenczy[12] and Benson and Sneeden[13] suggested that obesity is not associated with adenomyosis as opposed to the results of our study.
In our study, it is understood that patients with adenomyosis are multiparous compared with the control group patients. Many studies[1,3,4] reported that adenomyosis is common in multiparous women in parallel with the results of our study. In the study conducted by Lee et al.,[14] it was stated that 90% of the patients with adenomyosis were multiparous. Bergholt et al.[15] suggested that there was no significant difference between the number of parity and adenomyosis in their study, and this result is not compatible with the results of our study.
Another group of patients with increased risk of adenomyosis is patients with previous cesarean section.[5] In the present study, no significant association was found between adenomyosis and cesarean section, and this finding is consistent with the study of Riggs et al.[6]
The significant increase in the presence of leiomyoma in the control group was thought to be due to the demand for pelvic MRI examination to support the diagnosis of leiomyoma following ultrasonography. Koike et al.[10] indicated the association between the presence of adenomyosis and leiomyoma and did not correlate with the results of our study. There are reports that uterine leiomyomas should be considered in the differential diagnosis of focal adenomyosis.[4]
An inadequate number of histopathological confirmation is an important reason for the limitation of our study. However, histopathological diagnosis of adenomyosis can be confirmed in all five cases and may indicate high validity in the remaining cases. Another limitation is that the patients who were included in the study group were not questioned in terms of family history and no genetic analysis was performed in these patients.
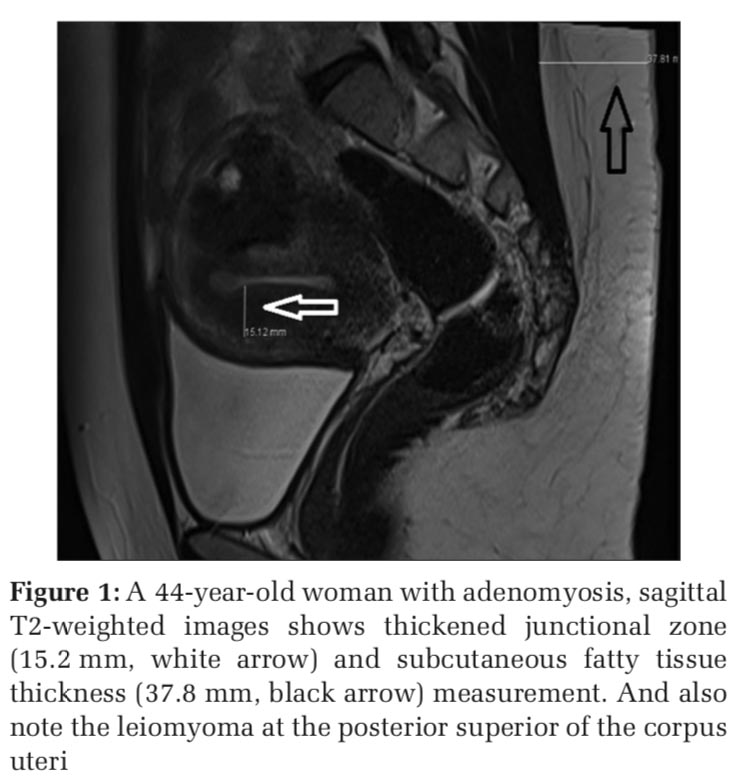

Adenomyosis is a common disease in which the etiology is unknown, but many factors are blamed. Obesity and consequently an increase in thickness in the subcutaneous fatty tissue may be one of the major risk factors for adenomyosis development.
Subscribe now for latest articles and news.