

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.v11.i3.25.51
Year: 2025, Volume: 11, Issue: 3, Pages: 268-274
Original Article
Kruti Karde1 , B C Koner1 , Binita Goswami1 , Nita Khurana2 , Deepti Goswami3 , Smita Kaushik1
1Department of Biochemistry, Maulana Azad Medical College, New Delhi, India,
2Department of Pathology, Maulana Azad Medical College, New Delhi, India,
3Department of Obstetrics and Gynaecology, Maulana Azad Medical College, New Delhi, India
Address for correspondence: Smita Kaushik, Department of Biochemistry, Maulana Azad Medical College, New Delhi, India.
E-mail: [email protected]
Received Date:08 February 2025, Accepted Date:16 April 2025, Published Date:20 October 2025
Background: Ovarian cancer is the third most common cancer of the female genital tract in females in the global population. CA125 is the most widely used diagnostic serum biomarker of ovarian cancer but is not specific. Osteopontin (OPN), a cell attachment molecule and Folate Receptor Alpha (FRα), a molecule that helps transport Folate across the cell membrane, are over-expressed in some cancers, including ovarian cancer.
Objective: The present study is designed to evaluate OPN and FRα as potential biomarkers for diagnosing ovarian carcinoma.
Method: Twenty-five patients with epithelial ovarian carcinoma, 25 patients with benign ovarian tumor and 25 healthy subjects as controls were enrolled in this study. Serum CA125 and Folate levels were measured using ECLIA, and serum OPN and FRα levels were measured using ELISA. Their levels were compared in 3 groups, ROC was used to determine the optimum cut-off of these markers.
Results: Optimum cut-off (Sensitivity & specificity) of CA125, OPN & FRα to differentiate ovarian cancer from healthy controls was 27 U/mL (92% & 92%), 3390.4 pg/mL (64% & 64%) and 149 pg/mL (80% & 72%) respectively; similarly to differentiate ovarian cancer from benign ovarian tumour it was 52.5 U/mL (76% & 76%), 3527.4 pg/mL ( 64% & 60%) and 283.5 pg/mL, in that order. The area under the curve of ROC when CA125 & FRα were combined was more than either of them individually.
Conclusion: FRα as a biomarker in adjunct to CA125 might improve the chance of diagnosis of ovarian cancer.
Keywords
Ovarian Cancer, FRα, FOLR1, Osteopontin, OPN, Markers
Ovarian cancer is the third most common cancer of the genital tract in females globally and the fourth among Indian females. 1, 2 It most often originates from the epithelial cells but may also originate from germ cells and stromal cells. 3 In a study published by Chaturvedi M. et al. 4 on the Indian population, roughly 89% of the ovarian cancers identified were epithelial in origin. Due to non-specific symptoms and lack of specific biomarkers, ovarian carcinoma is often diagnosed in advanced stages when prognosis is poor. 5
Currently, CA125 is the most commonly used biomarker for the diagnosis of Ovarian Carcinoma. A value <35 units/mL is taken as normal, while >35 units/mL is suspicious. However, raised CA125 has also been associated with benign conditions of ovaries 6 as well as in conditions apart from ovarian masses such as abdominal tuberculosis 5 , thus making CA125 a non-specific marker for ovarian carcinoma. In a meta-analysis by Dodge JE. et al., the sensitivity and specificity of CA125 were found to be 78% each. 7 Other non-specific markers associated with ovarian cancers are Human epididymis protein 4 (HE4), Carcinoembryonic Antigen (CEA), Cancer antigen 19-9 (CA 19-9), etc.
Osteopontin (OPN) is a calcium-binding protein expressed in bones, kidneys, and the brain, among 8, 9 It is also known as bone sialoprotein I (BSP-1 or BNSP), early T-lymphocyte activation (ETA-1), and secreted phosphoprotein 1 (SPP1). Although the precise functions of OPN are unknown, studies have suggested its role in cell attachment and signalling. 9 It has been found to be increased in various cancers such as colorectal, breast and small lung cell carcinoma. 10 Studies have also evaluated its role as a diagnostic marker of ovarian cancer. 11
Folate, being a part of one-carbon metabolism, is important for the synthesis of various molecules, including purines and pyrimidines and hence DNA synthesis and cell multiplication. 12 Folate is transported across the cellular membrane in three ways- through the reduced folate carrier (RFC), through the proton-coupled folate transporter (PCFT), and through folate receptors, of which there are four glycopolypeptide members (FRα, FRβ, FRγ and FRδ). 13 FRα has been reported to be overexpressed in solid tumours such as ovarian, lung and breast carcinomas.14 Given the important role of folate in cancer, aside from methotrexate and other drugs targeting folate, targeted therapy against the Folate receptor Alpha is also a subject of further investigation under the context of precision medicine.15, 16
In this study, we evaluated OPN and FRα as diagnostic marker for ovarian carcinoma.
This study was conducted in a tertiary care hospital by the Department of Biochemistry in collaboration with the Departments of Obstetrics and Gynaecology, and Pathology. The study was approved by the Institutional Ethical Committee (F.No.17/IEC/MAMC/2018/02, dated 26.10.2018). It was a hospital-based case-control study. Written informed consent was obtained before sample collection prior to surgery. Three groups of participants were recruited as follows:
Group I (Epithelial Ovarian Cancer Patients): The study included newly diagnosed treatment-naive patients with epithelial ovarian carcinoma aged between 20 and 70 in this group. Blood samples were collected before and after surgery (sample collection mentioned below), and patients who were confirmed to have cancer histopathologically were included in group I. In contrast, those with benign histopathology were considered for group II.
Subjects were recruited consecutively on admission in the wards till the sample size was met.
Group II (Benign Ovarian Tumor Patients): Patients in the similar age group as group I diagnosed with benign ovarian tumors as confirmed by histopathological examination of biopsy samples.
Group III (Control subjects): Clinically normal healthy women belonging to similar age group, without any ovarian abnormality as seen on ultrasound were included along with group II as controls.
Subjects with concurrent cancer at any other site, with present or past history of any bone diseases or with any other major systemic illness were excluded from the study.
Peripheral venous blood samples (5ml) in a plain vial were collected from
group I subjects by venipuncture, before surgery and 1 week post-surgery; samples from group II subjects were also collected similarly.
Samples from healthy controls were taken only once.
The study group was subjected to a structured questionnaire (regarding demographic, lifestyle and medical information). A detailed history of the onset of the disease and the presentation was taken, and a physical examination was carried out. A histological assessment of the tumour after surgery was done which included histopathological grade and type. Clinical staging was done using the FIGO classification, and histopathological typing and grading were done using the WHO classification.
Quantification of serum CA125 and Folate was done by Electrochemiluminescence immunoassay using an Elecsys kit adopted to Cobas e411 equipment (Roche Diagnostics, manufactured by Hitachi High Technologies Corporation, Tokyo, Japan).
The serum OPN and FRα were quantified using the Boster Picokine Human osteopontin Pre-Coated ELISA kit and the Picokine Human FRα Pre-Coated ELISA kit, respectively, provided by Boster Bio, CA. The assay was carried out using a multimode Perkin Elmer ELISA reader (VICTOR Nivo model, 940 Winter Street, Waltham, Massachusetts, 02451, USA).
Qualitative data were expressed in percentages. Statistical differences between the proportions were tested by Pearson’s chi-square test.
The numeric data was transformed into variables, coded and entered in Microsoft Excel and analysed and statistically evaluated using Statistical Package for the Social Sciences (SPSS)-PC-22. Kolmogorov-Smirnov test was used for checking normal distribution, and non-parametric data was expressed as median and interquartile range. The data was compared by using one way ANOVA or Kruskal Wallis H test followed by Bonferroni corrected post-hoc Mann-Whitney U test. Repeated measure data was compared using the Wilcoxon Signed Rank test. ROC Curve was plotted to identify the optimum cut-off value of biomarkers. The sensitivity and specificity of the cut-off were also calculated. Spearman’s correlation coefficients were used to examine the association between different biomarkers. A ‘p’ value <0.05 was considered as statistically significant for all statistical tests.
Most of the ovarian carcinoma cases belonged to the age group of 50-60, constituting 40% of study subjects. The mean age of cancer subjects was 49.72 years. The mean age of patients with benign ovarian tumour was 41.04 years, and of healthy subjects was 41.2 years. No significant difference in distribution concerning age was found in all the groups (p= 0.06).
Subjects in the three groups were further classified according to their menopausal status (Table 1). Among cancer subjects, 8 patients were premenopausal, and 17 patients were postmenopausal, which was found to be significant (p=0.014) when compared with benign and healthy controls.
Similarly, the parity status was significantly (p<0.014) different in the three groups. There were 20 multiparous women in the cancer group, 16 in the Benign ovarian disease group and 9 in the control group. Also, the control group had maximum nulliparous women (n=11) when compared with the cancer group (n=2) and benign ovarian tumor group (n=7) (Table 1).
Histopathological typing of ovarian tissue showed that most of the patients had serous malignancy (n=14) followed by mucinous adenocarcinoma (n=10) and seromucinous (n=1). Most subjects in the benign tumor group were of mucinous cystadenoma (n=10) followed by serous cystadenoma (n=7), dermoid cyst (n=5), endometriotic cyst (n=2), and Sertoli cell tumor (n=1).
|
|
Healthy Controls (n=25) |
Patients with Benign Ovarian tumours (n=25) |
Patients with Ovarian Cancer (n=25) |
p Value (Using ANOVA*, Chi-square test**, Fisher Exact test # |
|
Age (in years) |
41.2 |
41 |
49.7 |
0.06* |
|
Premenopausal
Postmenopausal |
18 (72%) 7 (28%) |
16 (64%) 9 (36%) |
8 (32%) 17 (68%) |
0.014** |
|
Nulliparous Single Child Multiparous |
11 (44%) 5 (20%) 9 (36%) |
7 (28%) 2 (8%) 16 (64%) |
2 (8%) 3 (12%) 20 (80%) |
<0.014# |
(a) Pre-surgery:
Estimation of baseline levels (Pre-surgery) of CA125, OPN, FRα and Folate are presented in Table 2.
|
Parameter |
Healthy Controls (n=25) |
Benign ovarian disease (n=25) |
Ovarian Cancer (n=25) |
|
Serum CA125 (U/mL) |
12.90(8.85-41.2) |
39.2(16.25-52.50)* |
295.3(59.95-799.3)*# |
|
Serum OPN (pg/mL) |
2483(1379-3680) |
2216(889.2-4663) |
4095(1421-5044) |
|
Serum FRα (pg/mL) |
54(0.1-199.1) |
212.2(22.33-337.8)* |
397.1(153.4-1794)*# |
|
Serum Folate (ng/mL) |
9.0(5.66-12.25) |
4.32(3.47-8.0)* |
5.15(4.3-6.0)*# |
|
*p < 0.05 compared to healthy controls, # p < 0.05 compared to Benign ovarian disease by Kruskal Wallis test followed by Bonn Ferroni corrected post-hoc Mann Whitney U-Test. |
|||
No significant difference was found in the median values of serum OPN in the three groups.
A significant difference in median values of FRα and CA125 was found in the ovarian cancer group when compared to the benign ovarian tumor group and control group. Serum folate level was significantly lower in both ovarian cancer and benign ovarian tumor group in comparison to controls, however, it was within normal reference range (5-20 ng/mL).
(b) Post Surgery:
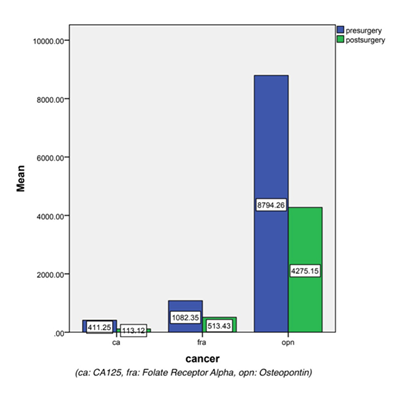
Follow-up of Group I & II patients: Levels of serum biomarkers were compared in the subjects before and after surgery (Figure 1) using 2-tailed Wilcoxon Signed Ranks test in cancer subjects and benign subjects. There was a significant (p<0.05) decrease in serum levels of Folate receptor Alpha (FRα) and CA125 post-surgery in cancer subjects; however, the decrease in serum Osteopontin (OPN) levels was insignificant (p>0.05).
In benign subjects, there was no significant difference in serum levels of OPN and CA125 before and after surgery, however, FRα decreased significantly post-surgery.
Spearman correlation showed a significant positive correlation between baseline levels of FRα and CA125 (r= 0.506, p=0.01) in cancer subjects, however, no significant correlation was found between OPN and CA125 (r=0.043, p=0.838).
Serum levels of FRα and CA125 were significantly increased in high-grade serous adenocarcinoma in comparison to mucinous adenocarcinoma, with p values of 0.019 and 0.02, respectively. However, OPN did not show any significant difference among different histopathological grading. None of the markers showed any significant difference with FIGO surgical staging.
To evaluate OPN and FRα as biomarkers for early diagnosis of ovarian carcinoma, ROC was plotted using pre-surgery or baseline serum values of OPN, FRα and CA125 between the groups (Table 3).
|
|
To differentiate benign from healthy controls |
To differentiate cancer from healthy controls |
To differentiate cancer from benign tumors |
|||
|
|
AUC (p value) |
Optimum cut off (Sensitivity & Specificity) |
AUC (p value) |
Optimum cut off (Sensitivity & Specificity) |
AUC (p value) |
Optimum cut off (Sensitivity & Specificity) |
|
CA125 |
0.834 (<0.001) |
16.75 U/mL (76% & 76%) |
0.939 (<0.001) |
27 U/mL (92% & 92%) |
0.84 (<0.001) |
52.5 U/mL (76% & 76%) |
|
Folate Receptor Alpha (FRα) |
0.676 (0.033) |
141.89 pg/mL (68% & 68%) |
0.851 (<0.001) |
148.98 pg/mL (80% & 72%) |
0.735 (<0.001) |
283.52 pg/mL (64% & 64%) |
|
Osteopontin |
0.504 (0.96) |
2602.74 pg/mL (52% & 48%) |
0.632 (0.109) |
3390.41 pg/mL (64% & 64%) |
0.614 (0.165) |
3527.4 pg/mL (64% & 60%) |
|
CA125+FRα |
0.834 (<0.001) |
- |
0.966 (<0.001) |
- |
0.851 (<0.001) |
- |
The demographic profile of cancer participants is presented in Table 1. As seen in the table, age distribution in the 3 groups was not different, hence, age is not a confounder while interpreting biomarkers in ovarian cancer. None of the subjects gave a history of use of oral contraceptives, which has been reported as a protective factor against ovarian cancer by several studies. 17 There was no significant family history of breast or ovarian cancer in these cases.
However, menopausal status and parity were different in the cancer group when compared with healthy controls and benign ovarian tumors. Most females in the cancer group were multiparous, despite the fact that increased parity 17, 18 is protective in ovarian cancer, which suggests that parity was not a confounding factor in the study. Most subjects (68%) of the ovarian carcinoma group were postmenopausal with an average of 37 years of reproductive years before menopause, compared to 36% of benign subjects and 28% of healthy controls. Menarche at an early age and menopause at a late age increases lifetime ovulatory years, which is a risk factor for ovarian carcinoma. 17
As shown in Table 2, basal serum levels of CA125 and Folate Receptor Alpha (FRα) were significantly higher in patients with benign ovarian tumors and patients with ovarian cancer when compared to healthy control; similarly, the difference was also significant between benign and ovarian cases. CA125 is a known biomarker for ovarian cancer; however, due to its limitations, there is a need for a different biomarker. With this study, we infer that FRα has the potential to be a biomarker in diagnosing ovarian cancer as its AUC was close to that of CA125; few studies have found that such potential exists. 19, 20 FRα has been studied with HE4 by Leung F. et al. and was found to be a strong predictor of ovarian cancer diagnosis. 20 It can distinguish between histological subtypes; a few studies have also demonstrated it being raised in high-grade serous ovarian histology. 21, 22 Studies have shown the therapeutic role of FRα 23, 24 and the role of intraoperative imaging of cells expressing it. 25, 26
Although FRα levels were high in benign and cancer groups, the serum folate levels in all the groups were within the reference range. Hence, the difference in FRα levels is probably not attributed to serum folate levels.
There was a significant decline in levels of CA125 and FRα after surgery in the cancer group; the decrease in OPN levels was, however, not significant (Figure 1). This again proves that CA125 and FRα are potential biomarkers of ovarian cancer. An increase in their levels during follow-up would be suggestive of a possible recurrence of cancer.
In the benign group, only serum levels of FRα showed a significant decline post-surgery.
There was a positive correlation between basal levels of CA125 and FRα.
Our ROC analysis shows that CA125 and FRα can be used to differentiate cancer from healthy controls and even from benign cases. For benign cases, AUC for FRα doesn’t improve much, but it increases significantly for cancer cases; thus, its discriminatory power increases (Table 3). There was a marginal increase in the Area under the curve when CA125 and FRα were combined to diagnose cancer from healthy and benign groups. So, we conclude that FRα in serum may act as an independent marker; in combination with CA125, its distinguishing ability increases.
Based on our study, CA125 is superior to FRα in sensitivity and specificity for the diagnosis of Ovarian Carcinoma. However, a combined study of the two markers with a bigger sample size is recommended to further evaluate its role in diagnosis.
With the surgical removal of cancer, there was a significant reduction in the levels of CA125 and FRα, suggesting a significant secretion of these biomarkers in serum by cancer cells. This finding can be explored further for monitoring cases of disease recurrence. In addition, increased expression of folate receptor alpha may reflect an increased demand for folate by the rapidly multiplying cancer cells.
ECLIA: Electrochemiluminescence assay
ELISA: Enzyme-linked immunosorbent assay
FRα: Folate Receptor Alpha
OPN: Osteopontin
RFC: Reduced Folate carrier
FIGO: International Federation of Gynecology and Obstetrics
Funding: None
Conflict of Interest: None
Subscribe now for latest articles and news.