Introduction
Chronic kidney disease (CKD) is a widespread health issue and its prevalence has risen in the past decade globally, attributed to an increase in the prevalence of lifestyle diseases and improved life expectancy. In India, the age-modified incidence rate of end-stage renal disease (ESRD) is 229 per million population (pmp), and >100,000 new patients come in renal replacement programs annually. 1
CKD is defined by a reduced estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2 body surface area or presence of albumin in urine for more than 3 months, which is recognized as a marker of increased glomerular permeability.2 Cerebrovascular and cardiovascular diseases are more common among patients with chronic kidney disease in comparison to the broad population.
CKD especially in advanced stages is associated with increased risk for stroke; which is six times higher in comparison to rest of the population and associated with higher mortality.3 Brain infarcts usually occur while or shortly after dialysis procedures, whereas haemorrhage is usually reported 35.5 hours after the last dialysis. The incidence of brain infarct was found to be more than brain haemorrhage. Elevated serum creatinine by itself is a marker for generalized vascular disease and a strong predictor of outcome after stroke. 4
Renal replacement therapy is accompanied by an increased risk of subdural hematoma, intracranial haemorrhage, and Wernicke’s encephalopathy; apparently due to hypertension and use of anticoagulation along with hemodialysis. 5
Small vessel disease (SVD) of the brain is a malady of clinical and radiological findings that results from abnormalities in perforating cerebral arterioles, capillaries, and venules. CKD has been associated with subclinical vascular disease in the form of subclinical brain infarcts and increased carotid intimal medial thickness. Although subclinical brain infarcts, intimal medial thickness, and white matter hyper intensities (WMH) have different risk factor profiles, they may all represent markers of systemic vascular disease and inflammation. The radiological findings include changes in the white matter and subcortical grey matter, small subcortical infarcts, lacunes, periventricular & deep white matter hyperintensity (PVH & DWMH), and cerebral microbleeds (CMBs). SVD of the brain is in league with increased risk of cognitive decline, dementia, mood disturbance and stroke. 6
SVD of the brain and glomerular small vessel disease might have a common origin of pathogenesis and hence be nearly related to each other. The kidney and brain are end organs with low-resistance which bear high volume blood flow throughout the cardiac cycle. Juxtamedullary afferent arterioles and perforating arteries in brain are evolutionary similarly developed to maintain perfusion of vital organs such as nephrons and brain directly from large arteries to deliver blood to tissues; these vessels are exposed to high blood pressure. Similar vascular factors may be responsible to cause damage to both the organs. Due to these hemodynamic analogy in vascular beds of the brain and kidney, SVD of the kidney may be indicative of SVD of the brain and vice versa. 7
Keeping this perspective, the current study was performed, with an aim to correlate the severity of ischaemic changes in the brain on MRI, with the grading of CKD. The relation between the severity of chronic kidney disease and ischaemic changes in the brain is not well documented and needs to be addressed in Indian patients.
Material and Method
This was a cross-sectional observational study conducted in a tertiary care centre with approval from an institutional ethical committee (S No. IEC/VMMC/SJH/Thesis/October/2016-76). Written informed consent was obtained from the patients. The study was conducted on sixty-five subjects aged > 18 years whose eGFR was less than 60 ml/min/1.73m2 for more than 3 months, on the basis of random sampling; out of which ten were on hemodialysis. Patients having acute renal failure, collagen vascular disorder, malignancy, post-renal transplant patients, cerebral AV malformation, symptomatic cerebrovascular disease prior to CKD, acute coronary syndrome, previous history of myocardial infarction, history of oral contraceptive pills intake and patients having contraindications for MRI were excluded from the study. The staging of CKD is as described in Table 1. 8
|
Stages of CKD |
Description |
eGFR (ml/min per 1.73m 2 ) |
|
1 |
Kidney damage with normal or increased GFR |
≥90 |
|
2 |
Kidney damage with mildly decreased GFR |
60-90 |
|
3A |
Mildly to Moderately decreased GFR |
45-59 |
|
3B |
Moderately to Severely decreased GFR |
30-44 |
|
4 |
Severely decreased GFR |
15-29 |
|
5 |
Kidney failure (end-stage renal disease) |
<15 or Dialysis |
CKD: Chronic Kidney disease; eGFR: Estimated Glomerular Filtration Rate
All the study subjects underwent an MRI of the brain on a 1.5-Tesla (Philips Achieva) scanner. The MRI protocol included the acquisition of T1W, T2W, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images (DWI). Gradient-echo T2W FLAIR (GRE) images were also acquired for analysis of cerebral microbleeds. The MRI acquisition parameters were T1W (TR/TE 594/15ms, min slice gap: 0.5mm), T2W (TR/TE 4862/110ms, min slice gap: 0.5mm), FLAIR (TR/TI 6000/2000, TE 120ms, min slice gap: 5mm) and T2*/FFE (TR/TE 702/23ms, without any interslice gap). Among these pulse sequences, T2*W(GRE) without the 180° refocusing pulse characteristic of spin-echo or fast spin-echo techniques is highly sensitive to the susceptibility effect. 9
The size and number of cerebral infarctions were noted and cortical infarcts were defined as infarcts involving cortical grey matter. Lacunar infarcts were defined as parenchymal lacks, not extending into the cortex, with a surrounding T2W hyperintense zone (3 mm-15 mm in diameter). (Figure 1) Subcortical infarct when size was more than 15 mm, but otherwise similar to lacunar infarcts. Silent brain infarction (SBI) was defined as an infarction more than 15mm in size that was evident on MRI but not accompanied by clinical symptoms.
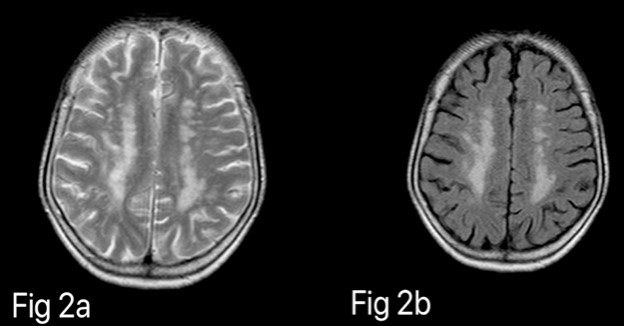
The extent of DWMH was noted and graded accordingly, best on FLAIR and T2WI. (Figure 2) WMHs were graded according to the Fazekas scale which divides the WMHs into periventricular and deep white matter hyperintensities (PVH & DWMH) and each is given a grade depending on the size and confluence of lesions. 10

The cerebral microbleeds (CMB) were defined as a focal area of size <10mm with a signal loss on T2WI that increases in size on GRE images (“blooming effect”) with clear boundaries and no oedema, hence, differentiating it from vascular flow void. (Figure 3) The areas of symmetrical hypo intensity in the basal ganglia likely representing calcification or non-haemorrhagic iron deposits were not taken into account. The CMB was recorded in three main regions, lobar (cortical grey matter and subcortical periventricular white matter), deep (deep grey matter, basal ganglia, thalamus, white matter of corpus callosum, internal capsule, and external capsule) and infratentorial (brain stem and cerebellum). The CMB was graded as Grade 1(1-5 microbleeds), Grade 2 (6-10 microbleeds), Grade 3 (11-15 microbleeds) and Grade 4 (>15 microbleeds). 11

Statistical Analysis
The data was entered in the MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0. A p-value of <0.05 was considered statistically significant. Categorical variables were presented in number and percentage (%) and continuous variables as mean ± SD and median. The normality of data was tested by the Kolmogorov-Smirnov test. If the normality was rejected then a non-parametric test was used. Quantitative variables were compared using the Unpaired t-test/Mann-Whitney Test between the two groups and Anova/Kruskal Wallis test between more than two groups. Qualitative variables were correlated using the Chi-Square/Fisher’s exact test.
Results
The study comprised a total of 65 patients; 39 males and 26 females, aged between 3 and 67 years (mean age: 36 ± 8 years). Most of the patients were of CKD stage 3B (46%) followed by stages 4, 3A and 5 (28%,17% and 9% respectively). Among these 10 patients who were on hemodialysis (7 males, 3 females; mean age 54.1 years) mean eGFR was 17.2 ml/min/1.73m2 and mean serum creatinine was 3.84 μmol/L. In 55 patients who were not on dialysis, the mean eGFR and serum creatinine was 36.4 ml/min/1.73m2 and 1.96 μmol/L respectively.
The study subjects were divided among two subgroups according to the presence/absence of SVD findings on brain MRI and the age, gender, and values of eGFR & S. creatinine were compared for any statistical significance. (Table 2) Also, the grading/number of these SVD findings was correlated with stages of CKD. (Table 3)
|
SVD findings on MRI Brain |
|
Age (yrs.) (Mean) |
Gender (Male/Female) |
eGFR (Mean) |
S. Creatinine (Mean) |
|
Lacunar infarcts |
Present(n=25) |
61.28 |
16-Sep |
26.4 |
2.65 |
|
Absent(n=40) |
43.72 |
20/20 |
37.7 |
2 |
|
|
P-value |
<0.001 |
0.269 |
<0.0001 |
0.002 |
|
|
Cortical infarcts |
Present(n=1) |
52 |
1/0 |
39.7 |
1.9 |
|
Absent(n=64) |
51 |
35/29 |
33.5 |
2.2 |
|
|
P-value |
0.979 |
1 |
0.591 |
0.872 |
|
|
Periventricular Hyperintensity |
Present(n=39) |
56.4 |
24/15 |
29.5 |
2.5 |
|
Absent(n=26) |
41.4 |
Dec-14 |
39.9 |
1.9 |
|
|
P-value |
0.001 |
0.222 |
0.0001 |
0.0003 |
|
|
Deep white matter hyperintensity |
Present(n=34) |
59.5 |
21/13 |
26.7 |
2.6 |
|
Absent(n=31) |
40.5 |
15/16 |
40.8 |
1.8 |
|
|
P-value |
<0.001 |
0.279 |
<0.0001 |
<0.0001 |
|
|
Cerebral Microbleeds |
Present(n=13) |
60.3 |
05-Aug |
23.5 |
2.6 |
|
Absent(n=52) |
48.2 |
31/21 |
35.8 |
2.1 |
|
|
P-value |
0.03 |
0.17 |
0.0004 |
0.02 |
SVD: Small vessel disease; MRI: Magnetic Resonance Imaging; eGFR: Estimated Glomerular Filtration Rate
|
SVD findings on MRI Brain (n=no. of patients with that finding)
|
Grade/Number of Ischemic findings
|
STAGES OF CKD
|
P-value
|
|||
|
3A
|
3B
|
4
|
5
|
|||
|
Lacunar Infarcts (n=25) |
1 |
0 |
6 |
2 |
0 |
<0.0001 |
|
2 |
0 |
3 |
6 |
2 |
||
|
3 |
0 |
1 |
1 |
3 |
||
|
4 |
0 |
0 |
0 |
1 |
||
|
5 |
1 |
0 |
0 |
0 |
||
|
Cortical Infarcts (n=1) |
|
0 |
1 |
0 |
0 |
0.757 |
|
Periventricular Hyperintensity (n=39) |
Grade 1 |
4 |
10 |
8 |
1 |
0.006 |
|
Grade 2 |
0 |
3 |
6 |
4 |
||
|
Grade 3 |
0 |
1 |
1 |
1 |
||
|
Deep white matter hyperintensity (n=34) |
Grade 1 |
1 |
13 |
12 |
3 |
<0.0001 |
|
Grade 2 |
0 |
0 |
2 |
3 |
||
|
Grade 3 |
0 |
0 |
0 |
0 |
||
|
Cerebral Microbleeds (n=13) |
1 |
0 |
1 |
3 |
1 |
0.157 |
|
2 |
0 |
2 |
2 |
2 |
||
|
3 |
0 |
0 |
1 |
0 |
||
|
4 |
0 |
0 |
1 |
0 |
||
SVD: Small vessel disease; MRI: Magnetic Resonance Imaging; CKD: Chronic Kidney disease
Lacunar infarcts were present in 25 patients (16 males, 9 females; mean age 61.2 years); the mean eGFR and serum creatinine of these patients was 26.4 ml/min/1.73m2 and 2.6 μmol/L with a p-value of < 0.0001 and 0.002 respectively. (Table 2) The presence and number of lacunar infarcts increased as the CKD stage advanced (p-value <0.0001). All the 6 patients of CKD stage 5 had lacunar infarcts. (Table 3) 80% of dialysis patients showed lacunar infarcts as compared to 30% of pre-dialysis patients (p-value 0.0001). Cortical infarct was found in only 1 patient with no statistically significant association with the stage of CKD.
PVH was found in 39 patients, who were significantly older than those without PVH. The prevalence was much higher in patients with high serum creatinine and a corresponding low eGFR with a p-value of 0.0001. (Table 2) PVH grade 1 was usually seen in CKD stages 3A & B, whereas grades 2 & 3 were seen in stages 4 & 5. (p-value 0.006) (Table 3) DWMH were seen in 34 patients with demographic characteristics tabulated in Table 2. In the lower CKD stages the DWMH grades were low and as the CKD stage advanced DWMH grades increased with a p-value of 0.0001. (Table 3) 90% of patients on dialysis showed PVH and DWMH as compared to 54% and 45% of pre-dialysis patients (p-value 0.032 and 0.0001 respectively).
CMBs were noted in 13 patients and S. creatinine & eGFR were significantly higher in these patients (Table 2). However, no significant correlation could be established between the prevalence & number of CMBs and stages of CKD (p-value 0.157). 30% of dialysis patients showed CMBs as compared to 18% of pre-dialysis patients; which was not statistically significant (p-value 0.721).
Discussion
In the present study, we assessed the prevalence of small vessel disease (SVD) of the brain (lacunar infarcts, PVH, DWMH and CMBs) in CKD patients which increased significantly as the CKD stage advanced and the changes were much more pronounced in patients who were on hemodialysis. Significant factors associated with SVD of the brain were older age, male gender, high serum creatinine and low eGFR, however, CMBs were seen more commonly in females.
In the present study, lacunar infarcts were seen in 25 patients (38.4%) and their number increases with the advancing stage of CKD. A significant association between age and the number of lacunar infarcts was also seen. Kobayashi et al. studied that lacunar infarcts were identified on magnetic resonance imaging (MRI) in 25% of patients with a creatinine clearance >40 mL/min/1.73 m2 and in 85% of patients with creatinine clearance <40 mL/min/1.73 m2; thus, the strongest contributing factor on multivariate analysis, for lacunar infarction was decreased creatinine clearance. 12 In another study conducted by Shima et al. on 324 pre-dialysis CKD patients, lacunar infarcts were identified on MRI in 108 (31.8%) patients. There was a significant association between the prevalence of lacunar infarcts and the CKD stage, with an increase in prevalence with the stage of CKD (p < 0.0001). 13
In the present study, it was seen that a decrease in eGFR is significantly correlated with an increase in the prevalence of PVH and DWMH as detected on the MRI brain. These lesions are considered a predictor of ischaemic or haemorrhagic stroke. This data was similar to a few previous studies which suggest that CKD can be a predictor of cerebrovascular events.
In a study conducted by Shima et al., out of 324 CKD patients, PVH was found in 174 (53.7%) and these patients were significantly older and had a history of hypertension, diabetes, smoking behaviour and peripheral arterial disease. Also, there was a significant association between the prevalence of PVH and the CKD stage, with a greater prevalence of PVH as the CKD stage advanced (p < 0.0001). 13 The present study also showed similar results with the presence of PVH in 60% of cases and DWMH in 52.3% of cases and an increase in these changes with increasing grade of CKD.
In the study conducted by Martinez et al., out of 52 patients with CKD (stage 3 or 4), white matter lesions were seen in 33% of cases. However, the stage of CKD was not associated with the presence of white matter lesions.14 While in the current study, PVH and DWMH were seen in 60% and 52.3% of cases respectively and a statistically significant correlation with the CKD stage was seen, which suggests that the renal dysfunction may be linked to arteriosclerosis in cerebral small vessels causing white matter hyperintensities. The variation in the percentage of patients showing white matter changes may be due to a wider age group in the present study (18-89 years) as compared to the former having an age group of 30-60 years.
The Northern Manhattan Study involved 615 stroke-free participants (mean age 70 years, 60% women). In multivariate analysis, creatinine clearance of 15 to 60 mL/min was in league with increased white matter hyperintensity volume (WMHV) (0.322; 95% CI, 0.095 to 0.550). Despite this, estimation of renal function was done and moderate-severe CKD was independently associated with greater WMHV, after adjusting for age and gender. 15
Ikram et al. investigated the relationship between kidney function estimated by eGFR and cerebral SVD by MRI brain; patients with lower eGFR had more DWMH and lacunar infarcts, while, latter was not found significant. eGFR values did not correlate with grey matter volume or lobar white matter volume. 16
Shima et al. found CMBs in 35 out of 162 CKD patients (25.6%); more prevalent in older age, males, hypertensives, and poor renal function. Also, there was a cogent increase in the prevalence of CMBs with the advancing stage of CKD (P < 0.01).17 However, in the current study, CMBs were found to be more prevalent in females of older age with poor kidney function and the presence of CMBs increased with progressing stage of CKD, however, the result was not statistically significant which could be attributed to the smaller sample size and another fact that we used FFE sequence instead of dedicated susceptibility-weighted imaging (SWI) sequences due to non-availability of the same. In another study on the general population, CMBs were found to correlate with old age, hypertension, smoking, leukoaraiosis, PVH and lacunar infarcts. 18
White matter hyperintensity (WMH) represents a subclinical form of leukoencephalopathy due to degeneration of small vessels. As compared to the normal population, CKD and hypertensive patients have a higher incidence of WMH.19, 20 The present study also reiterates the fact that WMH is more common in post-dialysis patients with 90% of dialysis patients showing these changes. Kurt et al. specifically investigated focal WMH and revealed that this type of lesion was more common in hemodialysis patients (56%) than in control subjects (27%). 21
In another study by Watanabe 22, 80 patients who underwent maintenance hemodialysis were evaluated by MRI and 35% had CMBs on T2*WI as compared to only 5% in asymptomatic or healthy elderly individuals. In the current study also, 30% of dialysis patients showed CMBs, however, the result was not statistically insignificant.
As in nature, nothing is perfect, the present study also had certain limitations. Since it was a cross-sectional study design, cause-effect relationship could not be established. Exact eGFR estimations from 24-hour urine collections with radio-labelled iothalamate could not be obtained because of the cost and resource constraints.
Finally, with this study results it can be predicted that as SVD of the brain progress, renal function in patients with CKD might deteriorate. Vice-versa, when renal function in patients with CKD declines, the SVD of the brain may progress and more concern is needed for the same. This implies the correlation between renal function and small vessel disease of the brain and its importance for management of these patients. This also highlights the marked importance of renal function as a decisive factor of cerebrovascular disease and/or a marker of cerebral small vessel disease.
Conclusion
Chronic kidney disease is increasingly recognised as a universal public health concern. The present study aimed to study the specific correlation between CKD and cerebrovascular disease including small vessel disease of the brain, as this issue has been relatively unexplored. In conclusion, we demonstrated that the SVD findings in the brain were more prevalent in older age patients with high serum creatinine and a corresponding low eGFR. Also, the number and grade of these SVD findings increased with advancing grades of CKD and these findings reinforce that CKD-associated pathological changes in small vessels of kidneys do exist in small vessels of the brain. Thus, CKD may be an independent and valuable predictor of cerebrovascular disease.
Funding
Nil.
Conflict of Interest
Nil.
Authors’ contributions
NB is the corresponding author, designed and revised the work, interpreted the data, and submitted the case. NB has approved the submitted version for publication. SKP has drafted the work and approved the submitted version for publication. BBT has revised the manuscript and approved the submitted version for publication. PR had provided the patients for the study and helped in designing the study. No disclosure. All authors read and approved the final manuscript.
Acknowledgements
We are thankful to the staff in the Department of Radiodiagnosis, VMMC and Safdarjung Hospital for their support and cooperation throughout the study.