

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.v10.i2.24.169
Year: 2024, Volume: 10, Issue: 2, Pages: 231-235
Case Report
Mala Mukherjee1 , Nikhil Era2 , Shatavisa Mukherjee3
1Department of Pathology, MGM Medical College and LSK Hospital, Kishanganj, Bihar, India,
2Department of Pharmacology, MGM Medical College and LSK Hospital, Kishanganj, Bihar, India,
3Department of Clinical & Experimental Pharmacology, School of Tropical Medicine, Kolkata, West Bengal, India
Address for correspondence:
Shatavisa Mukherjee, Department of Clinical & Experimental Pharmacology, School of Tropical Medicine, Kolkata, West Bengal, India. E-mail: [email protected]
Received Date:23 May 2024, Accepted Date:16 July 2024, Published Date:05 August 2024
Primary breast sarcomas are rare constituting <1% of all breast malignancies. These histologically heterogeneous non-epithelial aggressive neoplasm arises from the connective tissue within the breast and have a high tendency of recurrence even after excisional biopsy. Therefore, it necessitates a multidisciplinary approach to modify the overall prognosis and mortality. To throw light on this entity, we here report a few cases that presented as huge lumps in the breast and were missed in fine needle aspiration cytology. Each case was followed by modified radical mastectomy and was diagnosed as sarcoma of the breast confirmed by immunohistochemistry without any history of previous breast disease and radiation
Keywords: Sarcoma, Diagnosis, Fine Needle Aspiration Cytology, Immunohistochemistry
Breast sarcoma is a rare condition that occurs independently of other malignancies and without previous irradiation. The management of this condition remains controversial due to its infrequency and limited correlation between grading and outcomes. Advances in diagnostic methods, including immunohistochemistry, have improved identification accuracy. Breast sarcomas constitute a small fraction of all primary breast malignancies, originating from mesenchymal tissue.1 Due to the lack of agreement on the exact definition of breast sarcoma, different studies have varying criteria for inclusion, with some authors excluding cystsarcoma phyllodes because of the presence of both epithelial and stromal components. Breast sarcomas consist of different types, including angiosarcoma, malignant fibrous histiocytoma, stromal sarcoma, liposarcoma, leiomyosarcoma, dermatofibrosarcoma protuberans, osteosarcoma, fibrosarcoma, and rhabdomyosarcoma. Since primary breast sarcomas are rare and mostly documented through case reports or small series, the understanding of this entity is still limited. The management of primary breast sarcomas poses challenges, ranging from the absence of agreement on the ideal surgical approach and the necessity for adjuvant chemoradiation to the critical role of achieving an adequate resection margin. Overall, the prognosis for breast sarcoma is unfavorable. In this report, we present cases of primary stromal sarcoma of the breast with varying histological differentiations, shedding light on the diagnostic complexities involved in these cases
A 55-year-old lady gave a history of a palpable mass in the right breast for 3 years. She was nulliparous and had no family history of breast cancer. There was no history of breast implants or irradiation. On physical examination, the patient was pale. Breast examination revealed a huge, fungating, irregular mass involving the entire right breast. It was approximately 35 × 25 cm with areas of hemorrhage and necrosis (Figure 1 a). The right axillary lymph nodes were palpable. The contralateral breast was normal. Chest roentgenography, bone scintigraphy, abdominal ultrasound, and computed tomography of the chest, abdomen, and brain were all normal. Fine Needle Aspiration Cytology (FNAC) was done which showed a moderate cellular smear with malignant ductal cells in small loose cohesive clusters showing mild pleomorphism (Figure 2 a) in a background having an absence of bare bipolar nuclei giving an impression of invasive ductal carcinoma of the breast. The patient underwent a right mastectomy with axillary lymph node sampling. The postoperative course was uneventful. Microscopic examination showed spindle to oval cells with atypia and mitotic figures with large areas of necrosis (Figure 2 b, c, d). Some areas showed entrapped atrophic breast parenchyma. Normal and abnormal mitotic figures were seen in the range of 8–9/high-power field. IHC showed that all epithelial markers were negative except for vimentin (mesenchymal differentiation) (Figure 2 e), and there was a weak reaction for smooth muscle antigen; all other mesenchymal markers tested were negative. Lymph nodes showed extensive tumor deposits with large areas of necrosis. The tumor was diagnosed as undifferentiated primary stromal sarcoma with extensive lymph node metastasis.
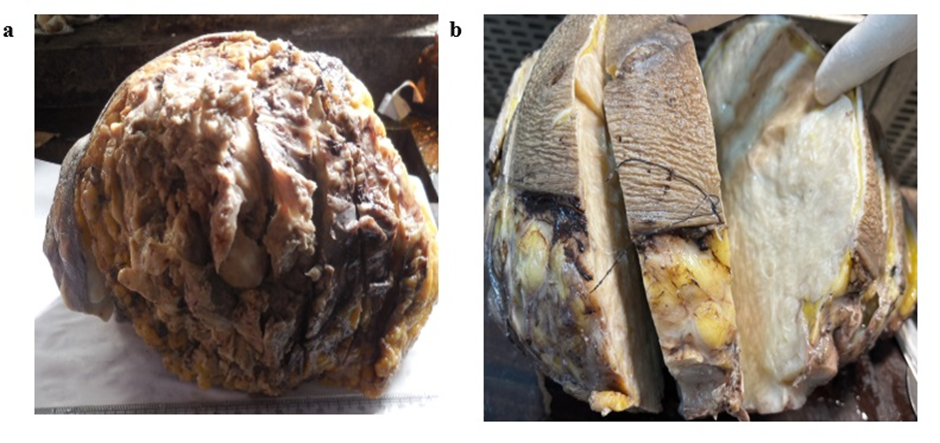

A 60-year-old female patient presented to the surgical department with a complaint of progressive swelling of the left mammary gland without pain. There was no history of previous breast trauma, bleeding, or family history of breast cancer. On examination, there was a single 7 x 5.5 x 3.5 cm mass, firm and non-tender in the upper right quadrant. There was a slight retraction of the overlaying skin. FNAC was done which showed only fibro myxoid component (Figure 3 a) with dispersed spindle cells which gave an impression of phyllodes tumour. An excision biopsy was done to confirm the case. Grossly, the cut section revealed a firm, discreetly lobulated, infiltrative unencapsulated gray-white tumor, measuring 7 x 5.5 x 3 cm. (Figure 1 b) There were no necrotic areas, areas of hemorrhage, or calcification. Surgical margins were tumor-negative.
Microscopically, hematoxylin and eosin (HE) stained sections revealed whorls and nests of spindle cells with mild differences in cellular size. The nuclei were round to oval with marked hyperchromasia (Figure 3 b) There were neither prominent nucleoli, nor cytoplasmic granules. Focally, tumor cells were round to oval with abundant cytoplasm, separated by delicate fibrous bands with lymphocytic infiltrates, and infiltrating adipose tissue. The tumor was found to be vimentin-positive and epithelial markers negative. The tumor was diagnosed as undifferentiated primary stromal sarcoma.

A 55-year-old female presented with a painless lump in her right breast measuring 6 x 4 x 3 cm firm, fixed with ulceration without any lymphadenopathy, and urgently sought a surgical opinion. Lump aspiration revealed malignant cells in loosely cohesive clusters in the background (Figure 4 a) absence of bare bipolar nuclei giving an impression of suspicion for ductal carcinoma and mastectomy was advised. Histology showed complete excision of a high-grade stromal breast sarcoma. Sections showed malignant spindle cell tumors exhibiting marked pleomorphism with osteoid matrix (Figure 4 b) osteoclastic giant cells (Figure 4 c) and frequent mitotic bodies. Osteoid differentiation (Figure 4 d) was also noted. Diagnosis of primary sarcoma of breast having osteoid differentiation was made.

A 38-year-old female presented with a painless lump in her left breast measuring 7 x 4 x 3 cm firm, fixed without any lymphadenopathy, and urgently sought a surgical opinion. Aspiration of the lump revealed low cellularity composed of malignant cells in loosely cohesive clusters in a myxoid background with an absence of bare bipolar nuclei (Figure 5 a) giving an impression of suspicion of malignancy and mastectomy was advised. On histology, sections showed malignant spindle cells in fascicles, and whorls (Figure 5 b) exhibiting marked pleomorphism and frequent mitotic bodies (Figure 5 c). No glandular component was seen. Some of the tumor cells had epithelioid morphology with giant cells (Figure 5 d). IHC- vimentin was positive (Figure 5 e). Diagnosis of primary sarcoma of the breast having leiomyosarcoma-like features was made.

Breast cancer is a highly common cancer type that mostly affects females. It has two primary forms, namely carcinoma and sarcoma. Carcinomas arise from the epithelial component of the breast, while sarcomas, which are rare, develop from the stromal (connective tissue) components of the breast. Primary breast sarcomas are very rare, and only a few hundred cases have been documented in the literature so far. 2, 3, 4, 5
Primary breast soft tissue sarcomas tend to affect women in their fifth or sixth decade of life. Male cases make up less than 5% of these tumors. Reported 5-year survival rates range from 14% to 91%. Possible risk factors include previous radiation exposure therapy, chronic lymphedema, preexisting fibroadenomas, and genetic conditions like neurofibromatosis or Li-Fraumeni syndrome 1, although more research is needed to confirm these associations.
Unilateral breast mass with rapid size increase and discoloration of the overlying skin is the typical presentation of primary breast sarcoma. FNAC can detect malignancy most of the time, but alone may not be sufficient in all cases. This is especially true for larger masses with varying consistency. In such cases, it should be followed by core biopsy if a diagnostic dilemma arises. Core biopsy is thus crucial for accurate diagnosis owing to the substantial size and atypical presentation of the mass. Fine needle aspiration is not accurate enough for categorization, though it can give a diagnosis of malignancy, and excisional biopsy should not be performed until other diagnostic attempts have failed.6, 7, 8 In our study, all cases initially underwent FNAC, but due to the mass's increased size, the diagnosis was inconclusive. As a result, further histopathological examination was performed for each case.
The American Joint Committee on Cancer staging system 9 is commonly used for staging breast sarcomas in soft tissue sarcoma. This system takes into account different variables including histological grade, tumor size, lymphatic involvement, and distant metastasis.10 These tumors tend to recur and have a poor prognosis. In four cases, the diagnosis was missed in FNAC due to the presence of a large tumor mass. Among them, two were initially thought to be invasive ductal carcinoma, one was suggestive of malignancy, and one was thought to be phyllodes. However, on histopathological examination, all four cases were found to be sarcoma of the breast.
FNAC may not always provide representative tissue samples, leading to potential false diagnoses.
It's recommended to complement FNAC with histopathology and immunohistochemistry for a conclusive diagnosis. This can be achieved with the preparation of a cell block, on which immunohistochemical evaluation may be performed for definite categorization. The treatment approach for breast sarcomas follows a multidisciplinary model, but there's currently no definitive agreement. Mastectomy without axillary lymph node dissection is the preferred treatment, with axillary dissection avoided unless there are clinically positive nodes. 11, 12 Achieving a negative surgical margin is a crucial determinant for both local recurrence and overall survival in these cases. High-risk cases may benefit from adjuvant or neoadjuvant chemotherapy, as well as radiotherapy. These treatment options should be taken into consideration. Several factors, including tumor grade, size, and histological type, affect the overall prognosis. Diligent follow-up is essential, as tumors measuring less than 5 cm are associated with a better outcome, underscoring their significance. In our study, all tumors were larger than 5 cm, and only one case could be followed, which presented with tumor recurrence.
Diagnosing sarcoma of the breast through FNAC is a challenge due to its rarity. Although spindle cells with nuclear atypia are suspicious findings on the smears, definite diagnosis may be missed due to the similarity with entities like phyllodes. So, all FNACs should be complemented by cell block or followed up with biopsy for histopathology and IHC to understand its line of differentiation for definite diagnosis and better prognostic outcome.
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
The study has been permitted by the institutional ethics committee for its possible publication (vide IEC-CH/234), concealing the patient identity.
Nil.
None Declared.
Subscribe now for latest articles and news.