

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2021.v07i01.013
Year: 2021, Volume: 7, Issue: 1, Pages: 73-80
Original Article
Roniya Francis1, Shruthi N Shetageri2, A N Roopa3, S R Raja Parthiban4
1Third Year Post Graduate, Department of Pathology, MVJ Medical College and Research Hospital, Bengaluru, Karnataka, India,
2Associate Professor, Department of Pathology, MVJ Medical College and Research Hospital, Bengaluru, Karnataka, India,
3Associate Professor, Department of Pathology, Shri Atal Bihari Vajpayee Medical College and Research Institute, Bengaluru, Karnataka, India,
4Professor and HOD, Department of Pathology, MVJ Medical College and Research Hospital, Bengaluru, Karnataka, India,
Address for correspondence:
Dr. S R Raja Parthiban, Professor and HOD, Department of Pathology, MVJ Medical College and Research Hospital, Dandupalya, 30th KM Milestone, NH-75, Kolathur Post, Hoskote, Bengaluru, Karnataka, India. Phone: +91-9845096970. E-mail: [email protected]
Background: Thrombocytopenia is one of the most common causes of abnormal bleeding and is defined as platelet counts < 1.5 lakhs/cumm. Three processes can cause thrombocytopenia, namely: Deficient platelet production, accelerated platelet destruction, and abnormal pooling of the platelets within the body. Of these, accelerated platelet destruction is the most common cause for thrombocytopenia and has variety of etiologies. The usefulness of bone marrow analysis in assessing accelerated platelet destruction is still debated. Therefore, a new simple and non-invasive diagnostic approach for thrombocytopenia is needed. Aims and Objectives: The present study was done with an aim to evaluate the use of platelet indices, namely, mean platelet volume (MPV), Platelet Distribution Width (PDW), and Platelet Large Cell Ratio (P-LCR) in differentiating the various causes of hyperdestructive thrombocytopenia.
Materials and Methods: This was a prospective study conducted over a period of 2 years and consisted of 206 cases of hyperdestructive thrombocytopenia. After recording relevant clinical details, platelet count along with platelet indices – MPV, PDW, and P-LCR was recorded. Based on the etiopathology identified, cases were categorized into three groups: Group I: Immunologic – cases of Immune thrombocytopenic purpura (ITP), Group II: Non-immune: Cases of sepsis and other non-immune causes of platelet destruction, and Group III: Viral and parasitic infections. Platelet indices were compared between the study groups and the control group which included 100 healthy individuals. Comparison was done among the three study groups as well.
Results & Conclusions: Dengue accounted for the highest number of 131 (89.72%) cases in the study. MPV, PDW, and P-LCR were significantly higher (P < 0.0001) when compared to the healthy controls except P-LCR in Group II. A statistically significant increase in MPV was noted among ITP cases when compared to other causes of thrombocytopenia. There was no difference in PDW and P-LCR among the study groups. To conclude, among the three platelet indices, MPV appears to be a good parameter in differentiating immune from other causes of hyperdestructive thrombocytopenia.
KEY WORDS: Hyperdestructive thrombocytopenia, mean platelet volume, platelet distribution width, platelet count, platelet large cell ratio.
Platelets also known as “thrombocytes” are small anucleate blood components which play an important role in maintaining vascular integrity and hemostasis. Platelets were first discovered by Giulio Bizzozero way back in 1882.[1] A platelet count < 1.5 lakhs/cumm is defined as thrombocytopenia which is the most common cause of abnormal bleeding. Clinical symptoms vary ranging from easy bruising to life-threatening, based on the severity of thrombocytopenia. With counts < 10,000/ cumm, a peak in spontaneous mucosal bleeding including intracranial and gastric bleeding has been documented in the literature.[2]
Thrombocytopenia results primarily from three mechanisms: Deficient platelet production, accelerated platelet destruction, and abnormal pooling of the platelets within the body. Hyper destructive thrombocytopenia is largely due to extramedullary platelet destruction. This subsequently leads to stimulation of thrombopoiesis in the marrow and thereby increases the number, size, and rate of maturation of megakaryocytes. Being the most common cause of thrombocytopenia, it is grouped into immune and non-immune pathologies.[3]
It is very important to know the cause of thrombocytopenia for correct patient management whereby unnecessary invasive procedures, transfusions, and medications can be avoided. The usefulness of tedious bone marrow examination in the evaluation of accelerated platelet destruction is debatable. Therefore, a new non-invasive approach to the diagnosis of thrombocytopenia is necessary.
The introduction of various indices into automated hematology analyzers has revolutionized the understanding of various hematological disorders including platelet disorders.[4] Platelet parameters such a mean platelet volume (MPV), platelet distribution width (PDW), and platelet large cell ratio (P-LCR) appear to be new potential biomarkers in several diseases due to their easy availability and inexpensive measurement methods.[5] However, the efficacy of these underutilized parameters in diagnosing the underlying platelet disorders needs to be established for clinical use.
Aims and objectives The present study was done with an aim to evaluate the use of platelet indices, namely, MPV, PDW, and P-LCR in differentiating the various causes of hyperdestructive thrombocytopenia.
This prospective study was conducted at the Department of Pathology, MVJ Medical College and Research Hospital, Bengaluru, over a period of 2 years (August 2018-2020) following Institutional Ethics Committee Clearance. Study included 206 consecutive cases of hyperdestructive thrombocytopenia.
Blood samples were collected in ethylenediamine- tetra-acetic acid (EDTA) anticoagulant tube and analyzed using Sysmex XS-800i. Samples were analyzed within 3 h of venipuncture to eliminate the possibility of platelet swelling in EDTA. Thrombocytopenia cases were identified on a daily basis from the hemogram reports generated in the hospital laboratory. Platelet count and platelet indices (MPV, PDW, and PLC-R) were recorded.
Patients with platelet count < 1.5 lakhs/cumm and confirmed by peripheral smear examination were included in the study. Thrombocytopenia due to decreased bone marrow production, artifactual thrombocytopenia and those for which hematology analyzer did not provide results of platelet indices were excluded from the study. Results of special tests performed for determining the etiology of thrombocytopenia such as bone marrow examination, serology for dengue, sepsis profile, and quantitative buffy coat along with other relevant clinical details were collected from the case files.
Based on the etiology identified, thrombocytopenia cases were categorized into three groups:
Group I: Immunologic – cases of immune thrombocytopenic purpura (ITP)
Group II: Non-immune: Cases of sepsis and other non-immune causes of platelet destruction
Group III: Viral and parasitic infections
Study also included 100 healthy controls who were age- and sex-matched. Adult blood samples primarily included from pre-employment health checkups and patients undergoing minor elective surgeries. Similarly, healthy newborn blood samples were also included in the control group. Platelet indices were compared between the study and the control group. Comparison was done among the three study groups as well.
Statistical analysis
The data were analyzed using Microsoft Excel and SPSS software version 24. Descriptive data were expressed as mean ± standard deviation. The Paired t-test and one-way ANOVA were used for comparison between the groups. P < 0.05 was considered statistically significant.
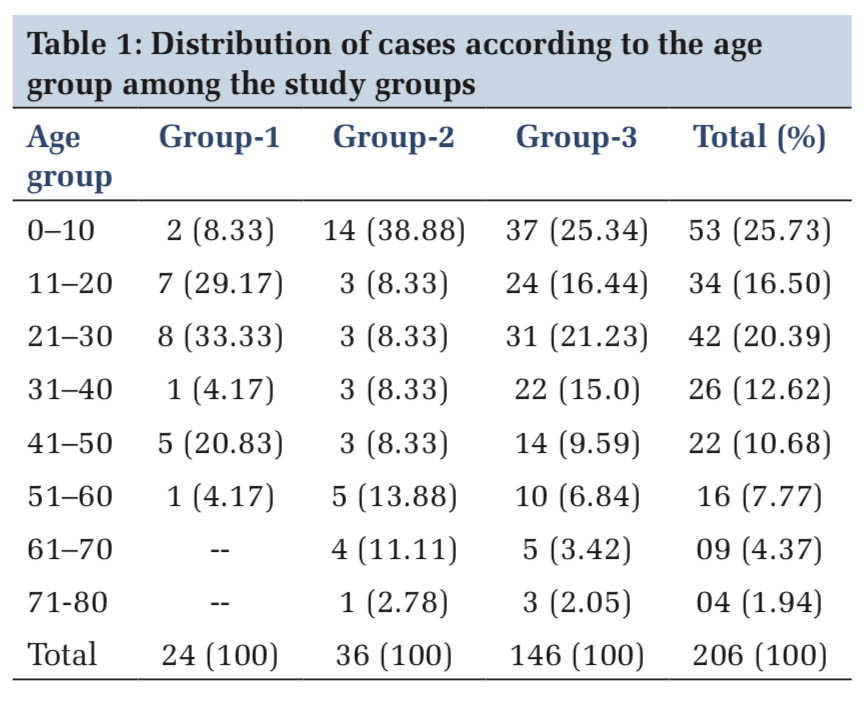
A total of 206 cases were included in the study. Out of which, 126 were males and 80 were females with a male to female ratio of 1.57:1. The age of the patients ranged from 2 days to 72 years with a mean age of 28 years.
The maximum number of cases belonged to 1–10years accounting to (53) 25.70% of cases, followed by (42) 20.39% cases in the age group of 21–30 years (Table 1).
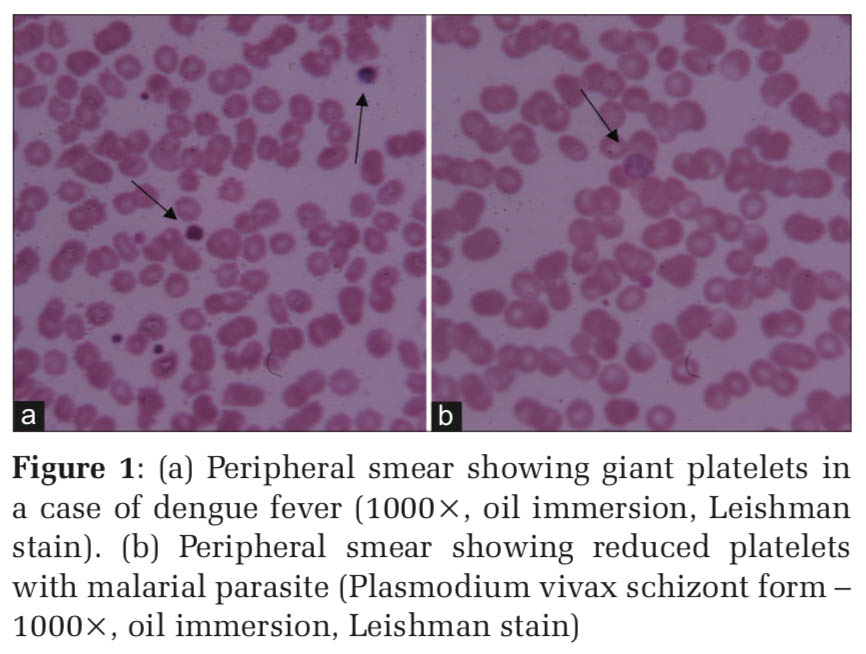
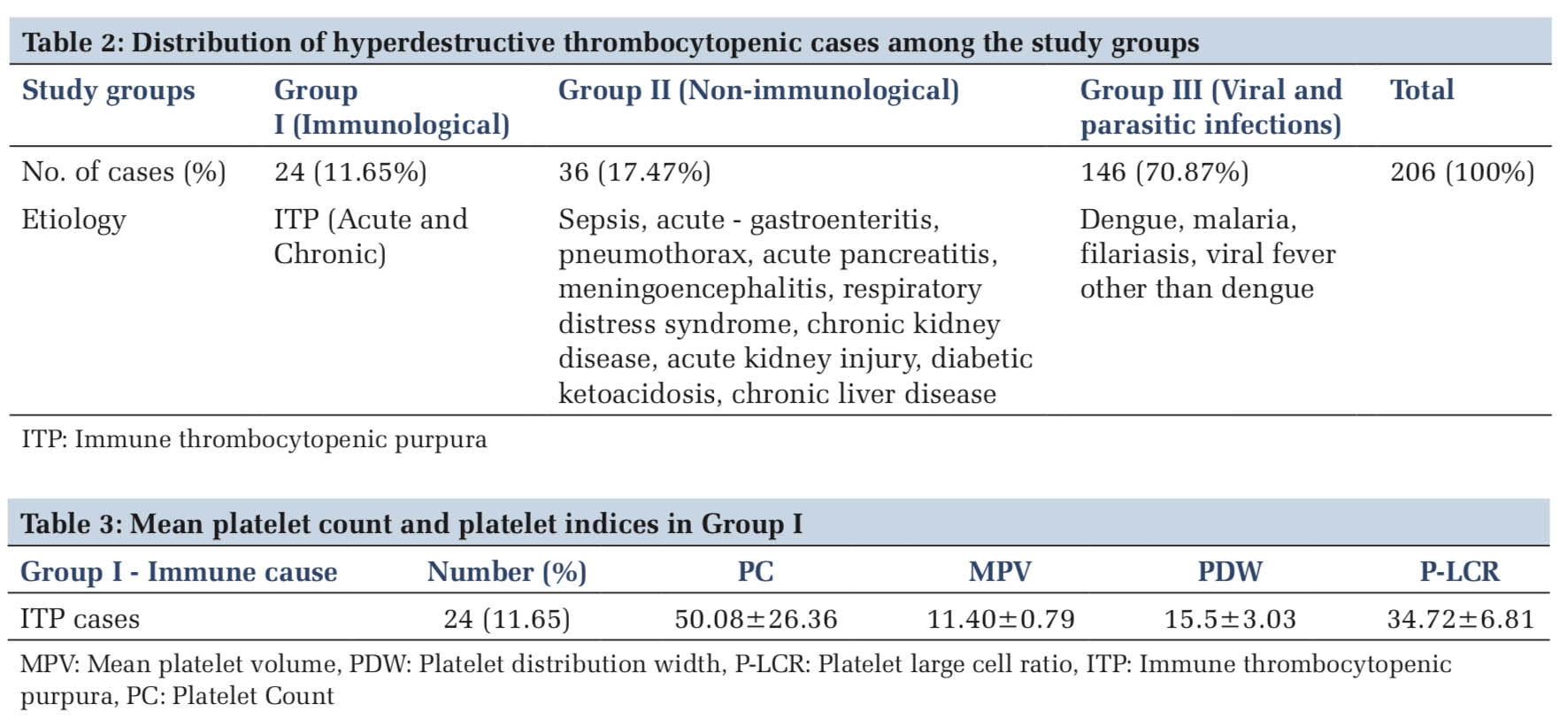
The etiological distribution revealed, most of the thrombocytopenia 146 (70.8%) cases were due to infective cause (Table 2). Mean platelet count and platelet indices of individual cases are summarized in Tables 3-5. Dengue accounted for the highest number of 131 (89.72%) cases (Table5 and Figure 1a).
The mean age in Group 1, Group 2, and Group 3 was 28.16 years, 25.32 years, and 27.48 years, respectively. Fourteen (58.33%) cases of ITP cases were females. Sepsis was seen commonly in newborn and pediatric age group.
Both MPV (11.94 ± 0.79) and P-LCR (41.02 ± 6.65) were highest in thrombocytopenia cases due to viral fever and lowest in acute pancreatitis (MPV- 9.5 ± 0.0) and (PLCR – 26 ± 1.90) belonging to Group II.
PDW (18.32 ± 4.90) was observed to be highest in malaria cases (Figure 1b) while the lowest in Scrub Typhus (13.8 ± 0.84).
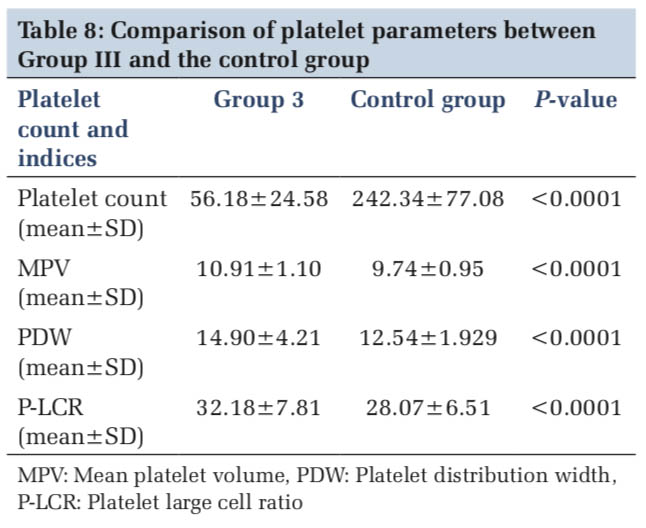
The mean and standard deviation of the platelet parameters in each group were studied and compared with the control group and in between the groups (Tables 6-8).
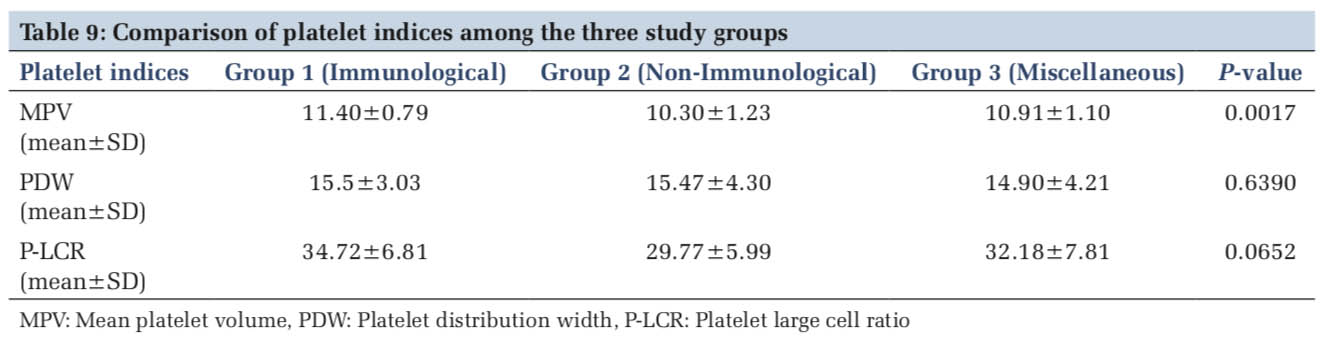
When the platelet indices were compared among the three study groups, only MPV showed a statistically significant increase in Group I cases comprising of ITP. There was no significant difference in PDW and P-LCR when compared among the three study groups (Table 9).
There are numerous studies in the literature which have evaluated the use of platelet indices in differentiating the hyper destructive thrombocytopenia from hypoproduction of platelets. However, there are fewer studies done to evaluate the utility of platelet indices in differentiating immune and non-immune causes of hyper destructive thrombocytopenia.
The automated hematology analyzers function based on the principles of electrical impedance, light scatter, and fluorescence. Although the generated platelet indices in the automated analyzers can be really informative regarding platelet kinetics and the probable mechanism of thrombocytopenia, they are not yet widely accepted for routine clinical use.[6]
MPV is one such parameter that offers an estimate of platelet size and indirectly their activity. The average MPV in a healthy person is usually 8.4–12.0 fL. Larger platelets are more enzymatically and metabolically active than smaller platelets and are represented by an increase in MPV. In hyper-destructive thrombocytopenia, there is a compensatory increase in platelet production along with release of immature platelet forms into the circulation. As immature platelet size is larger than the size of mature ones, the MPV measured by an automated blood analyzer is usually higher than in the case of bone marrow abnormalities.[4]
PDW like Red cell distribution width represents the degree of heterogeneity of cells. Studies done on MPV and PDW have shown a good sensitivity and specificity in the diagnosis of thrombocytopenia.[7,8] Normal PDW ranges from 8.3 to 14.0 fL. Platelet activation which causes an increase in the number and size of pseudopodia affects the estimated PDW values.[4]
P-LCR is another marker of platelet activity that estimates the percentage of all platelets with a volume more than 12 fL in the circulation. It normally ranges between 15% and 35%.[5] Elsewefy et al.[9] suggested that P-LCR was significantly higher in cases of ITP and values >33.6% helped in diagnosing these cases with a diagnostic accuracy of 99.60%.
Automation has its own drawbacks as seen in routine work where the platelet indices are not always recorded by the instrument. Commonly encountered conditions causing these difficulties include cases of severe thrombocytopenia, conditions with red cell fragmentation, and EDTA-induced pseudo thrombocytopenia. In these conditions a platelet histogram cannot be adequately drawn, and indices cannot be recorded. Hence, in these kinds of scenarios, their use can be limited.[4] Review done by Pogorzelska et al.[5] in 2020 on 45 articles published on platelet indices suggested that different methods used in hematology analyzers (optical, impedance) and the calibrations of analyzers do affect the outcome of measurements thus highlighting the need of standardization in platelet indices measurement.
Our study included a total of 206 cases of thrombocytopenia. Maximum number of cases belonged to first and third decades with a mean age of 28 years. In the study done by Gulati et al.,[10] maximum number of cases belonged to first decade while Reddy et al.[11] reported maximum number of cases in third decade. The age of the patients ranged from 2 days to 72 years. The oldest case in our study was of a 72-year-old male who was diagnosed to have dengue fever with NS1 antigen positive while the youngest case was a 2-day old male child diagnosed with respiratory distress syndrome.
Our study showed a male predominance with M:F of 1.57:1. This was similar to the study done by Gulati et al.[10] with M:F of 1.56:1 and Yalavarthi et al.[12] with M:F of 1.13:1.
Infections (70.87%) accounted for the maximum number of cases in our study. Yalavarthi et al.[12] (85.5%) and Mala et al.[13] (60.2%) also reported infections to be the most common cause of thrombocytopenia in their studies. Dengue fever was the single most common cause of thrombocytopenia amounting to 63.1% of cases in our study. However, in studies conducted by Parveen and Vimal[14] and Gulati et al.,[10] dengue fever comprised only 30.85% and 25.3% of cases, respectively. Malaria cases were higher in other studies when compared to our study[10,14,15] (Table 10). This difference may be attributed to the demographic variations and endemicity of the infection (Figure 1a and b).
ITP cases accounted for 11.6% in our study. Bone marrow examination was done in 70% of cases (Figure 2a and b). MPV was significantly higher in ITP cases when compared with the control group of healthy individuals. It was also statistically significant when compared to other two study groups (P = 0.0017).
MPV and PDW were elevated in ITP cases in studies done by Bessman et al.,[16] Kaito et al.,[4] Ntaios et al.,[17] and Elsewefy et al.[9] which reflected an increase in the production rate (Table 11). They established MPV cutoff values to be ranging from ≥ 9 fl to ≥ 11 fl in hyperdestructive group. Study done by Norrasethada et al.[1] and Numbenjapon et al.[18] suggested a MPV cutoff of ≥8.8 fL and ≥ 7.9fl, respectively, for differentiating hyperdestructive and hypoproductive groups. However, Borkataky et al.[19] found no significant difference in MPV values between the hyperdestructive thrombocytopenia group and the control group.
PDW and P- LCR were also higher in ITP cases when compared to control group in our study. In a study conducted by Negash et al.,[20] a higher PDW (15.5 ± 3.2) and P-LCR (36.83 ± 13.0) values were seen in comparison to the control group (PDW [12.5 ± 1.70] and P- LCR [27.2 5.2]). Khan et al.[21] suggested that all ITP cases which had MPV>11 fl and PDW>14 fl to be sensitive and specific indicators for ITP. Sepsis cases accounted for 44.44% of the total Group II cases. Mean MPV in sepsis cases in our study was 10.35 ± 1.23 which was higher than the control. In a study done by Gao et al.[22] in china, comparing the platelet indices among survivors and nonsurvivors of septic shock, found that an increase in MPV, and to a lesser extent rise in PDW and P-LCR to be indicative of worse prognosis in septic shock patients. They further suggested that after comparing platelet indices with traditional prognostic markers of sepsis, MPV was found to be more closely correlated with mortality, only second to serum lactate dehydrogenase levels.
Our study showed a significantly higher MPV, PDW, and P-LCR among dengue patients when compared to the control group. Afsar et al.[23] also observed a significant increase in MPV values in dengue cases when compared to control group. Contrary to this finding, in a study done by Bashir et al.,[24] a decreased MPV and a high PDW were noted in dengue patients when compared to healthy controls. Mukker and Kiran[25] also had a similar observations of a low MPV and a high PDW in severe thrombocytopenia (< 20,000/cumm) cases when compared to higher platelet count groups. However, Sharma and Yadav[26] showed no significant correlation between MPV and severity, serology and treatment outcomes in dengue cases thereby refuting the role of MPV in dengue cases.
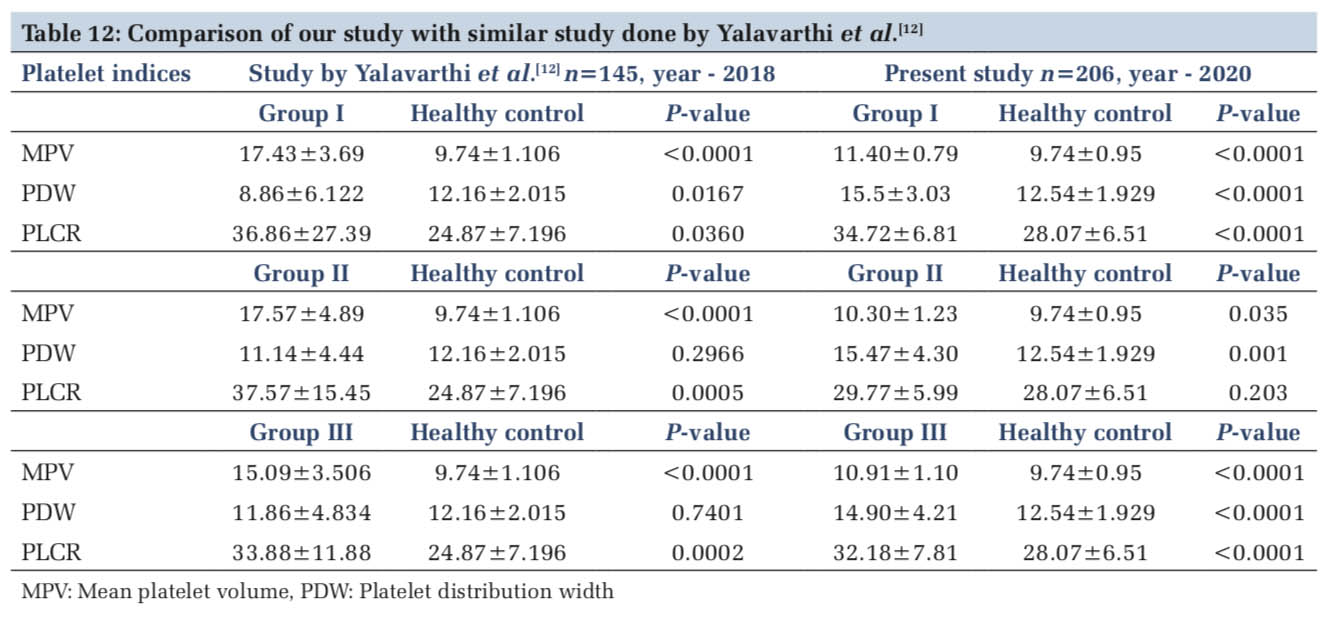
In a similar study done by Yalavarthi et al.,[11] MPV and P-LCR were significantly higher in study groups in comparison to healthy controls while there was no significant variation of PDW in non- immune thrombocytopenia and miscellaneous groups (Table12). They also found PDW to be significantly lower in ITP cases when compared to healthy controls. While in our study, MPV showed a significant increase in group I (ITP) cases when compared to other groups.



This study was conducted to understand the variation of platelet indices in different causes of hyper destructive thrombocytopenia. Proper evaluation and establishment of etiology in cases of thrombocytopenia are the first step towards patient management. Many studies in the past have analyzed the utility of these platelet indices in differentiating hyperdestructive from hypo proliferative conditions causing thrombocytopenia, but studies analyzing the utility of these indices within the hyperdestructive group are limited. The present study was done with this objective. The study highlights the variation of platelet indices in disease state compared to healthy controls where all the three parameters were significantly altered. Of the three indices, MPV shows promise as marker to differentiate immune causes from other cases of hyperdestructive thrombocytopenia. Studies including larger sample size along with a meta-analysis of various studies in the future are needed to prove the efficacy of these parameters for clinical significance.
|
Subscribe now for latest articles and news.