Anitha B Chalageri1, K G Arun2, T P Dinesh Kumar3, G B Vijaykumar4
1Consultant Pathologist, Columbia Asia Hospital, Mysore, Karnataka, India,
2Consultant Nephrologist, Columbia Asia Hospital, Mysore, Karnataka, India,
3Consultant Urologist, Columbia Asia Hospital, Mysore, Karnataka,India,
4Consultant Radiologist, Columbia Asia Hospital, Mysore, Karnataka, India
Address for correspondence:
Anitha B Chalageri, Consultant Pathologist, Columbia Asia Hospital, Bangalore, Mysore Ring Road Junction, Mysore, Karnataka,
India. Phone: +91-9480168021. E-mail: [email protected]
Abstract
Development of renal tumors is a main complication of acquired cystic kidney disease (ACKD). ACKD associated renal cell carcinoma (RCC) is the most common subtype of RCC occurring in ACKD and is a recently established entity. ACKD associated RCC occurs exclusively in patients with ACKD and hence the name. The tumor is characterized by cells with eosinophilic cytoplasm, cribriform/sieve-like architecture, and extensive intratumoral oxalate crystals. We report, a case of ACKD associated RCC in a 35-year‑old female with end-stage renal disease with ACKD on maintenance hemodialysis, who underwent left nephrectomy for massive hematuria and review the literature.
KEY WORDS:Acquired cystic kidney disease, complications, end-stage renal disease, hemodialysis, renal cell carcinoma
Introduction
Acquired cystic kidney disease (ACKD) is usually observed in patients with the end-stage renal disease (ESRD) undergoing long-term dialysis.[1] Neoplastic transformation is one of the major complications of ACKD.[1,2] ACKD associated renal cell carcinoma (RCC) is the most common subtype of RCC occurring in ACKD and is a recently established entity.[3,4] At the 2012 International Society of Urological Pathology consensus conference, 91% of respondents thought that ACKD associated RCC should be recognized as a distinct renal cell tumor entity and it has been included in the Vancouver classification in 2013.[5,6] The tumor is characterized by cells with abundant granular eosinophilic cytoplasm arranged in a sieve-like pattern with intratumoral calcium oxalate deposits.[1-8]
|
Case Report
A 36-year-old female with neurogenic bladder, secondary hypertension, and secondary vesico‑ureteric reflux, progressed to ESRD in December 2009. She was treated for tuberculosis in the past. She had mild adrenal insufficiency and was on alternate day steroids. She was started on hemodialysis (HD) through Jugular access in February 2010. She had primary arteriovenous fistula failure and was shifted to continuous ambulatory peritoneal dialysis (CAPD) from May 2010, doing three cycles daily. She developed recurrent peritonitis and was shifted back on HD through right brachiocephalic access from July 2014. She had recurrent painless hematuria and progressive anemia, for which she was evaluated and noted to have bilateral shrunken kidneys with multiple hemorrhagic cysts (Figure 1). She underwent left nephrectomy along with lymph node and peritoneal biopsy in January 2015. A month later, she presented with severe left-sided pain abdomen with hypotension and underwent emergency laparotomy and was noted to have splenic laceration with hemoperitoneum. She succumbed to refractory shock post-operatively.
Gross Received left nephrectomy specimen measuring 15 cm × 10 cm × 6 cm, two lymph nodes measuring 2 cm × 1 cm each and a bit of peritoneal biopsy. The surface showed numerous expanding cysts varying widely in diameter and some reaching up to 5 cm. On cut section, parenchymal atrophy, with numerous cysts, and fibrosis replacing original parenchyma were seen with disappearance of the corticomedullary junction (Figure 2). Cysts were filled with clear serous fluid and few were hemorrhagic. At one focus near the hilar region was seen a 1 cm × 0.6 cm brownish well-circumscribed area with tan colored solid tiny nodules within (Figure 2). The pelvis and the ureter were dilated. The ureter measured 8 cm long. Multiple sections were excised from grossly suspicious areas. Lymph nodes and peritoneal biopsy fragments were grossly unremarkable.
Microscopy Microscopically, a complex picture of end‑stage kidney disease consisting of chronic tubulointerstitial nephritis with thyroidization of tubules, severe vascular nephrosclerosis, oxalosis, focal hemosiderosis, microcalcification, and cystic spaces found. Lining of the cysts was variable from mostly flattened or low cuboidal epithelium to hobnail cells with eosinophilic cytoplasm. Few cysts showed atypical papillary hyperplasia and stratified epithelial projections.
Sections from the suspicious lesion near the pelvis showed tumor tissue with cystic spaces lined by epithelial cells with eosinophilic cytoplasm, papillary tufts, and cribriform epithelial proliferations with abundant intracellular hemosiderin (Figure 3). Few cystic spaces were filled with the hemosiderin‑laden macrophage. Nuclei were round to oval with prominent nucleoli (Fuhrman Grade 2-3) (Figure 3). Many intratumoral, intraluminal, and interstitial oxalate crystal deposits were seen (Figures 3 and 4). Renal vessels were uninvolved. Lymph node showed reactive changes and a peritoneal biopsy was unremarkable. Based on these findings ACKD associated RCC was reported.
|
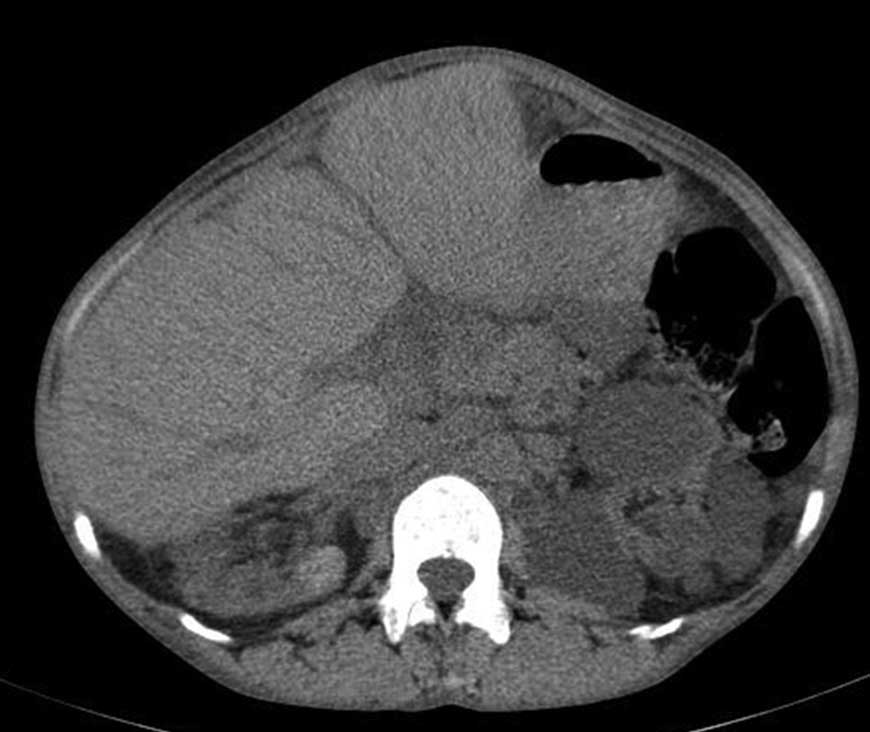 |
Figure 1: Computed tomography scan axial showing bilateral shrunken multicystic kidneys with hyperdense areas likely representing hemorrhagic cysts |
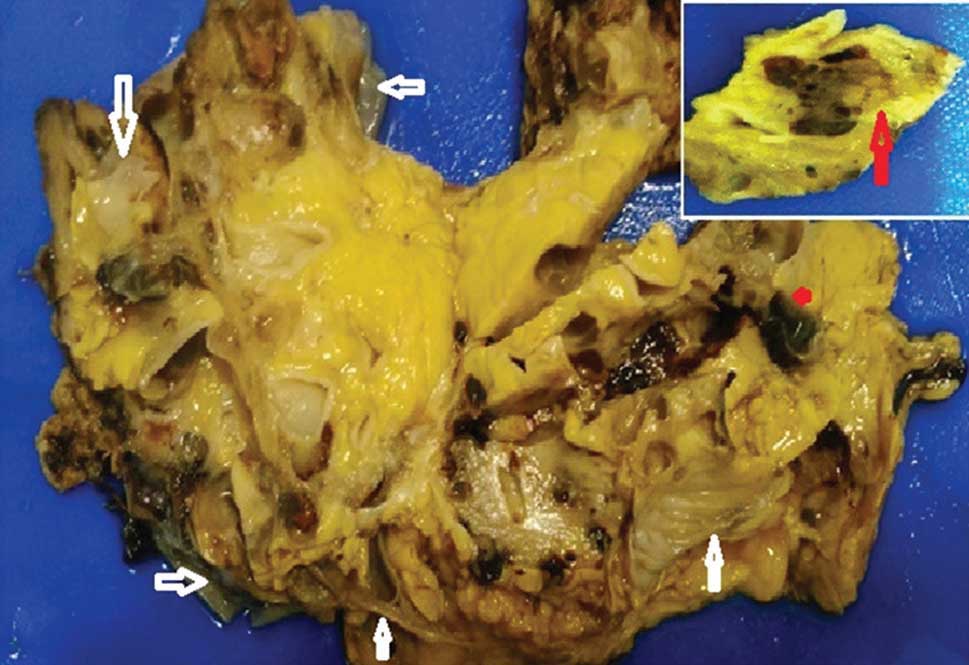 |
Figure 2: Gross appearance of the left kidney on the cut section with loss of architecture and multiple collapsed cysts (white arrows). Inset shows tumor focus (red arrow) |
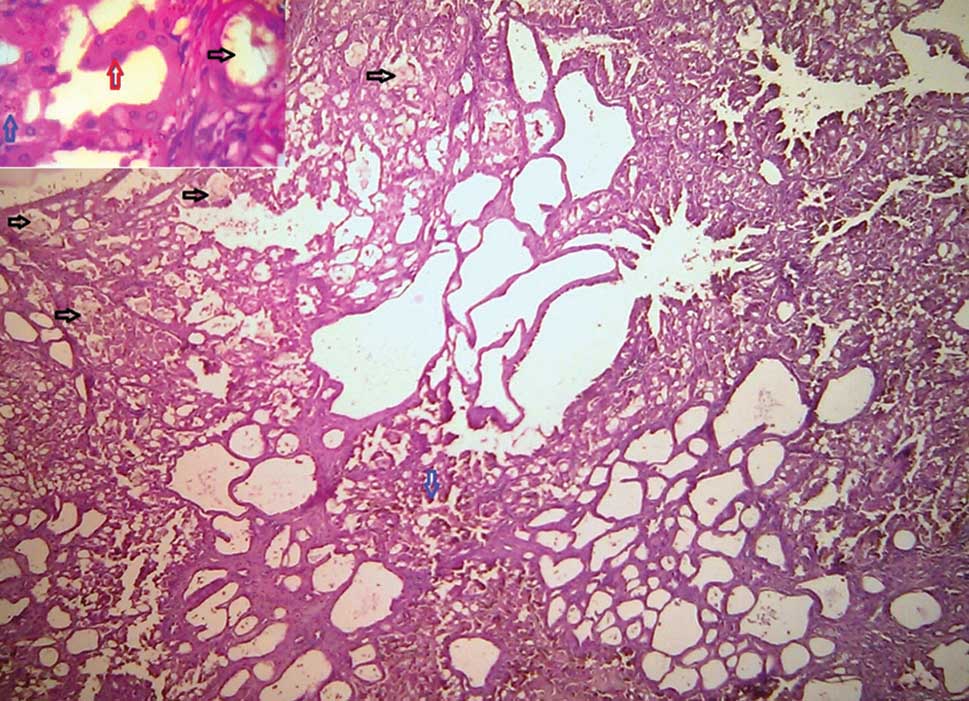 |
Figure 3: Tumor with sieve-like pattern, calcium oxalate crystal deposits (black arrows), hemosiderin deposition (blue arrows) (H and E, ×10). Inset: Abundant eosinophilic cytoplasm and Grade 2-3 nuclei (red arrow) and oxalate crystal (black arrow) (H and E, ×40) |
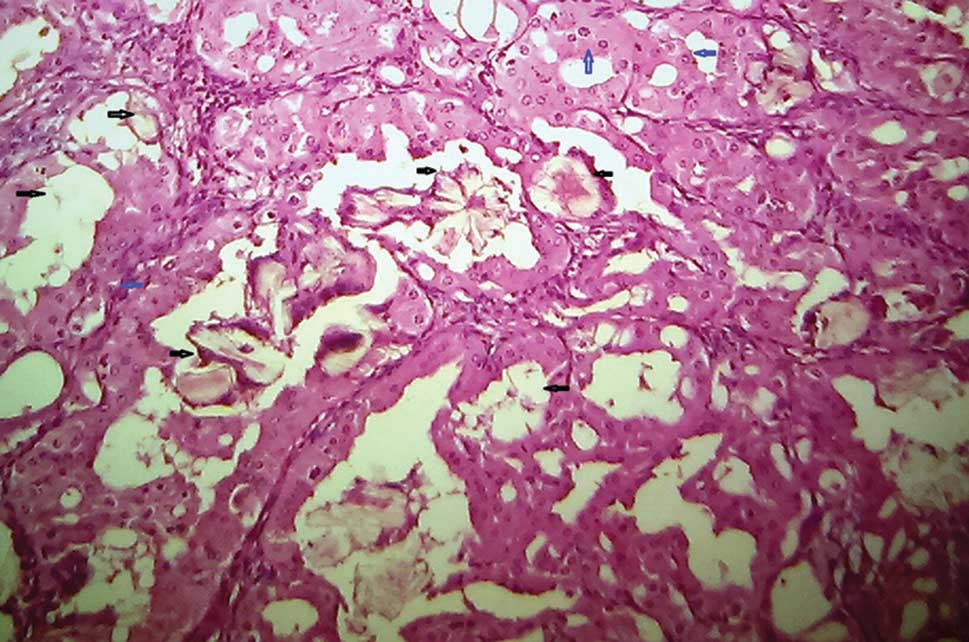 |
Figure 4: Eosinophilic cytoplasm, nuclei with prominent nucleoli (blue arrow) and oxalate crystals (black arrows) (H and E, ×40) |
|
Discussion
ACKD is the development of fluid-filled cysts in the kidneys of patients with ESRD due to non‑cystic kidney diseases. ACKD occurs as an an effect of ESRD in patients on maintenance HD.[1] ACKD is an important risk factor for the development of RCC not only in maintenance HD patients but also in the CAPD population.[8] Studies have shown that approximately one-third or more of patients on long‑term HD of more than 3 years will develop ACKD and 20-50% will eventually develop into RCC.[9] They occur approximately 20 years earlier than in the general population and are four to seven times more common in males than females.[10,11] Tumors in ACKD are frequently multifocal (50%) and bilateral (20%).[5,7]
Review of literature shows, RCC in a patient with ACKD may be asymptomatic in as many as 86% cases.[10,11] In symptomatic patients, the most common symptoms are due to bleeding into the tumor tissue, renal parenchyma into the subcapsular, and retroperitoneal spaces or into the pelvicalyceal system leading to persistent gross hematuria.[7,11] The occurrence of hematuria, flank pain or an unexplained fever or systemic illness in a dialysis patient is an indication for renal imaging.[11] However, in some cases, persistent hematuria may be the only symptom and the tumor may not be detectable by usual imaging studies, as renal parenchymal bleeding obscures the underlying tumor.[7,10,11] Our patient presented with massive hematuria with severe anemia.
ACKD-RCCs arise from cyst walls, filling the lumen completely, sometimes.[5,7] They can also arise as solid masses separate from the cysts.[5] Cut surfaces are tan to yellow with hemorrhagic areas.[5] Microscopy shows acinar, tubular, alveolar, microcystic, macrocystic, papillary, and solid sheet like patterns in varying combinations.[5,7] The tumor is characterized by cells with abundant granular eosinophilic cytoplasm with Fuhrman Grade 2-3 nuclei.[3-5,7] Intra- and intercellular microscopic lumina imparting a sieve-like pattern along with the presence of intratumoral oxalate crystals are diagnostic of this entity.[2-5,7] Background kidney shows ESRD features with ACKD.[7] Surrounding cysts are lined by similar tumor-like oncocytic cells and multilayering with papillary hyperplasia are seen frequently.[7]
ACKD-RCCs stain diffusely positive for alphamethylacyl- CoA racemase and negative or only focally positive for CK7.[7] However, a specific immunohistochemistry profile is not required to make the diagnosis.[7]
ACKD - Associated RCC has a relatively good prognosis as most cases are detected at an early stage. However, it is reported to have a relatively more aggressive behavior than other RCC subtypes.[3,7]
|
Conclusion
ACKD - Associated RCC is a distinct renal cell tumor entity with a relatively good prognosis when detected at an early stage. Hence, periodic screening of all ESRD patients on long-term dialysis is very important. A young and relatively fit longterm dialysis patient will be more benefitted than elderly patients if such tumors are detected at an early stage.
|
References
- Rioux-Leclercq NC, Epstein JI. Renal cell carcinoma with intratumoral calcium oxalate crystal deposition in patients with acquired cystic disease of the kidney. Arch Pathol Lab Med 2003;127:E89-92.
- Sule N, Yakupoglu U, Shen SS, Krishnan B, Yang G, Lerner S, et al. Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: A comprehensive study. Am J Surg Pathol 2005;29:443-51.
- Tickoo SK, dePeralta-Venturina MN, Harik LR, Worcester HD, Salama ME, Young AN, et al. Spectrum of epithelial neoplasms in end-stage renal disease: An experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol 2006;30:141-53.
- Kuroda N, Ohe C, Mikami S, Hes O, Michal M, Brunelli M, et al. Review of acquired cystic disease‑associated renal cell carcinoma with focus on pathobiological aspects. Histol Histopathol 2011;26:1215-8.
- Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) vancouver classification of renal Neoplasia. Am J Surg Pathol 2013;37:1469-89.
- Delahunt B, Srigley JR. The evolving classification of renal cell neoplasia. Semin Diagn Pathol 2015;32:90-102.
- Crumley SM, Divatia M, Truong L, Shen S, Ayala AG, Ro JY. Renal cell carcinoma: Evolving and emerging subtypes. World J Clin Cases 2013;1:262-75.
- Master U, Cruz C, Schmidt R, Dumler F, Babiarz J. Renal malignancy in peritoneal dialysis patients with acquired cystic kidney disease. Adv Perit Dial 1992;8:145-9.
- Hla OO, Pemasari Upali T, Ghazala K, Prathibha PS, Sowmya TR. Haemodialysis related renal cell carcinoma. Brunei Int Med J 2013;9:93-6.
- Truong LD, Krishnan B, Cao JT, Barrios R, Suki WN. Renal neoplasm in acquired cystic kidney disease. Am J Kidney Dis 1995;26:1-12.
- Blagg CR. Acquired cystic renal disease. Saudi J Kidney Dis Transpl 1997;8:105-12.

