

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2021.v07i01.006
Year: 2021, Volume: 7, Issue: 1, Pages: 32-37
Original Article
Callixte Yadufashije1, Mireille Ingabire1, Madjidi Sibomana1, Emmanuel Munyeshyaka1, Joseph Mucumbitsi1, Francois Niyonzima1, Albert Onyango Mala2, Georges Bahati Sangano3, Lydia Mwanzia4, Thierry Habyarimana1
1Department of Biomedical Laboratory Sciences, INES Ruhengeri Institute of Applied Sciences, Rwanda,
2Department of Medical Laboratory Sciences, Jomo Kenyatta University of Agriculture and Technology, Kenya,
3Department of General Nursing, School of Nursing, University of Rwanda, Rwanda, 4Department of Midwifery and Gender, Moi University, Kenya
Address for correspondence:
Dr. Callixte Yadufashije, Department of Biomedical Laboratory Sciences, INES Ruhengeri Institute of Applied Sciences, Rwanda. Phone: +250788273428. E-mail: [email protected]
Objective: This study aimed to evaluate the association with the oral microbial community derangement and dental disorders during pregnant.
Materials and Methods : A total of 60 pregnant women were selected, of the 60 women, 30 pregnant women had dental disorders, and the remaining 30 did not have dental disorders (control group). The oral swab samples were collected from the women during a routine clinic visit. Samples were transported to INES Ruhengeri Clinical Microbiology Laboratory for microbial identification analysis. Chi-square test (x2) was used to test association with oral microbial community imbalance and dental disorders. Data were analyzed by SPSS version 22.
Results Yeast was the most isolated microorganism among both groups of women and stood at 100% and 80%, respectively. The oral microbial community derangement was statistically significant (x2 =52.93, P < 0.00001) to contribute to dental disorders among pregnant women. For single microorganism derangement, Staphylococcus aureus (x2 = 5, P = 0.025347), Enterobacter spp. (x2 =7.4, P = 0.006522), and Staphylococcus spp. (x2 = 30, P < 0.00001) were statistically significant to contribute to dental disorders.
Conclusions: Pregnancy leads to oral microbiota dysbiosis which is a risk factor for dental disorders among pregnant women. Women should seek dental consultation during pregnancy for early detection and management of dental disorders.
KEY WORDS: Dental disease, dysbiosis, microbiota, oral, pregnancy.
The oral cavity is the main gateway for microorganisms to enter and reside in the human body and other animals. The greatest challenge has been to differentiate externally entered microorganisms and the oral normal flora microbial communities. Oral microbial community ensures the maintenance of the normal oral health of an individual. However, any microbial imbalance, maladaptation, and derangement in the oral microbiota lead to the development of oral diseases including dental infections.[1] With this regard, the most common known dental disorders are gingivitis, dental caries, glossitis, and other chronic or aggressive forms of periodontitis. Pregnancy status is associated with various physiological changes that predispose pregnant women to oral microbiota variation.[2]
The oral route contains a special microbial community with different microbiome compared to any other place of gastrointestinal tract. The association found between oral microbial community dysbiosis is dynamic and has an influence on different aspects such as nutritional habits, smoking, and others. In normal healthy conditions, oral microbiota enhances and takes part in a critical metabolism, physiological and immunological role.[3] The oral cavity harbors diverse microbes and more than 700 endogenous prokaryotes have been discovered. Among these types, 54% were given names, 14% are always identified through culture but not named, and 32% are referred to as uncultivable phylotypes. Isolation was done to only 280 species using the culture method and others were identified through gene-based cloning of 16s RNA.[4] Oral microbial compositions among healthy individuals were found to be of great significance to human health.[5]
Pregnancy period is a special state for women’s health and featured with the complexity of physiological changes. Various changes in physiological functions affect not only the reproductive system but also other parts of the body including the oral cavity. Pregnancy has also been a critical time to educate women about the prevention of dental caries during antenatal care in different health centers and hospitals around the world.[6] The relationship of periodontal infections was previously studied and pregnancy outcomes such as preterm delivery and low birth weight were observed to be associated with this oral health conditions.[7]
The oral cavity changes occurring during pregnancy have been a part of interest for reproductive biologists. Diverse effects from women physiological changes during pregnancy were identified as a result of oral microbiota maladaptation. Inflammatory responses and gingivitis were investigated and observed to contribute to the rise of estrogen and progesterone.[8] Health conditions such as gingivitis and periodontitis increase their prevalence rate during pregnancy, this is clearly due to various changes in physiology which also alter the oral microbiota protecting oral health.[9] More than half of pregnant women do not consult dentists during pregnancy. Many women do not mind on their oral health during pregnancy for their own protection as well as their fetus. The reason for this negligence should be due to the lack of adequate knowledge to their oral health and its correlation to health and disease for both the mother and the fetus.[10] This study aimed to analyze oral microbiota dysbiosis and its association with dental diseases among pregnant women.
Study area
This study was carried out at Gatenga health Centre located in Kicukiro district, Kigali city, Rwanda. Samples were taken from women attending antenatal care services at Gatenga Health Center.
Study design
This was a cross-sectional study and purposive sampling was used to select study participants. The study was conducted from October to December 2019.
Sample size
The study targeted pregnant women where a total of 60 women participated in the study. Of 60 pregnant women, 30 of them had dental disorders while other 30 participants did not have dental disorders.
Sample collection
Oral swab samples were collected and swabbing techniques were followed during specimens’ collections. Women who accepted to participate in the study signed an informed consent. Participants were invited and offered a seat in the swab collection room. The procedure was explained before samples were collected. Cotton swab, tubes, and normal saline were used for sample collection. For both groups of women, a cotton swab was used to collect the sample in dental surface and oral root and put in normal saline. The samples collected were immediately sent to the laboratory for analysis.
Laboratory analysis
Macroscopic of oral swab
Macroscopic examination was performed through observation of the swabs specimens under microscope.
Culture media preparation
Blood Agar (BA), MacConkey Agar (MCA), and Sabouraud Dextrose Agar (SDA) were all used and prepared by dissolving some calculated grams of the amount of the dehydrated culture media into 500 ml of distilled water based on instruction on culture media. Heated with repeated stirring and boiled from 1 min to 2 min for compete dissolution. The solution was autoclaved at 121°C for 15 min, and then cooled down to be distributed in petri dishes for culture.
Inoculation and incubation
Using pour plate method, inoculation was done to expose the bacteria to a well growing medium into well prepared Petri dishes of BA, MCA, and SDA media. The plates were incubated at 35–37°C for 18–24 h before examining the growth of different colonies on the petri dish.
Smear preparation and gram staining procedures
Before staining, the slide smear was prepared. A drop of normal saline was added to the slide and aseptically transfers a colony from the Petri dish. The culture was spread with an inoculation loop to an even thin film over a circle of 1–5 cm in diameter. The culture was fixed it over a gentle flame. Moreover, the solution of crystal violet was added over the fixed culture and waited for 60 s. The stain was poured off and gently rinsed the excess stain with a stream of water from a faucet. Then, the iodine solution was added to the smear to cover the fixed culture and waited for 60 s. The iodine solution was poured off and rinsed the slide with running water and shaking off the excess water from the slide. Furthermore, a few drops of decolorizer, ethanol, or acetone were added on the slide and rinsed it off with water for 5 s. Counterstain, safranin was flooded on the slide for 40–60 s and washed off the solution with water and making the slide air dried. Then, the stained smear was examined under a microscope.
Biochemical Test
Kligler iron agar test
An inoculating straight loop was used after sterilization in the blue flame of the Bunsen burner and then allowed to cool. A colony of the suspected organism from MCA and BA was picked, stabbed by the medium up to the butt of the KIA tube and then it was streaked back and forth along the surface of the slant. Again, the neck of the KIA was flamed, capped, and placed in the incubator for 18–24 h at a temperature of 37oC.
Catalase test
Two drops of 3% hydrogen peroxide were put on a clean glass slide using a dropper, a pure colony of the organism was picked from BA using a wire loop. Placed the colony on the hydrogen peroxide on the glass slide; emulsification was put into action. Observation for bubble formation was carefully done within 30 s.
Coagulase test
Dilute plasma from human blood was used with peptone water. A loopful of the test organism was put into the diluted plasma which made a complete suspension. Incubation of the suspension was done at a temperature of 37°C then examination for clot formation was made.
Indole and citrate test (IC) tests
ThegrownbacteriafromBAwereinoculatedinto tryptophan broth and incubated at 37°C for 24 h at room temperature. Kovac’s reagent was added to the broth culture and the red color formation occurred to show the presence of indole. For the side of citrate test, an agar slant with synthetic medium containing small amounts of mineral salts (citrate and ammonium) was used to perform the test. Bromothymol blue (pH indicator) was mixed with culture medium and then inoculation was done after 12–24 h, and if positive bacteria were able to use citrate which turned the indicator from green to blue.
Oxidase test
One colony of the suspect organism was transferred to a filter paper soaked with oxidase reagent (tetramethyl-p-phenylenediamine dihydrochloride). The blue color appeared within 10–15 s for the indication of positive results.
Statistical analysis
After laboratory work, data were cleaned and entered into Excel spreadsheet and analyzed using SPSS version 22. Tables and figures were used to present results. Chi-square test (x2) was used to test association between oral microbial community derangement and dental disorders among pregnant women. The level of significance was set at 0.05.
Ethical consideration
The permission to conduct the study was given by both Ruhengeri Institute of applied sciences ethical committee and Gatenga health center. Pregnant women were informed about the study before collecting samples. The right to privacy and confidentiality was respected.
Sociodemographic characteristics of participants As indicated in Table 1, among 60 (100%) recruited pregnant women, (15–20 [age were 16.6%, (20–30) age were 46.6%, (30–40)]) were 36.8%. The mean age of the population was 28 years and the range age was 24 years. The population regarding their marital status was 26.7% of single, 33.3% married, 40% divorced, and 0% widower. Regarding their education level, 43.3% had never gone to school, 16.6% had completed primary studies, 33.4% did secondary studies, and 6.7% did university.
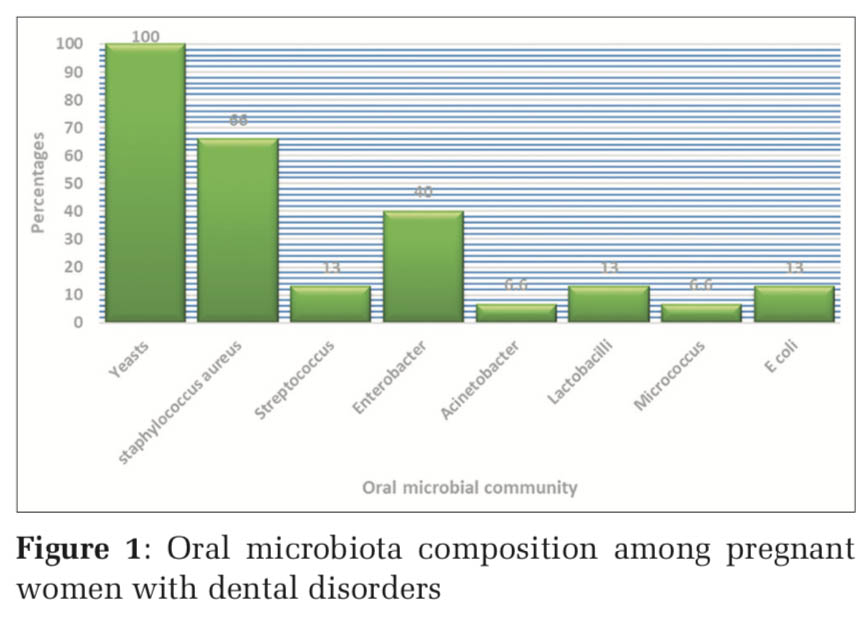
Oral microbial community composition among pregnant women with dental disorders
The predominant oral microbial community composition was the yeast (100%) which occurred in all women with dental diseases. Staphylococcus aureus (66%) was the second predominant microorganism and Enterobacter (40%) was the third. Other microorganisms were found as follows Lactobacillus (13%), Streptococcus (13%), Escherichia coli (13%), Acinetobacter (6%), and Micrococcus (6%). Acinetobacter and Micrococcus were the least identified oral microbial community. Figure1 shows the identified oral microbiota composition among women with dental disease.
Oral microbial community composition among pregnant women without dental disorders
Yeast (80%) was the predominant followed by Staphylococcus spp. (66%) and the last isolated microorganism was S. aureus (13%). Figure2 indicates the findings regarding the identified oral microbial communities among women without dental disorders.
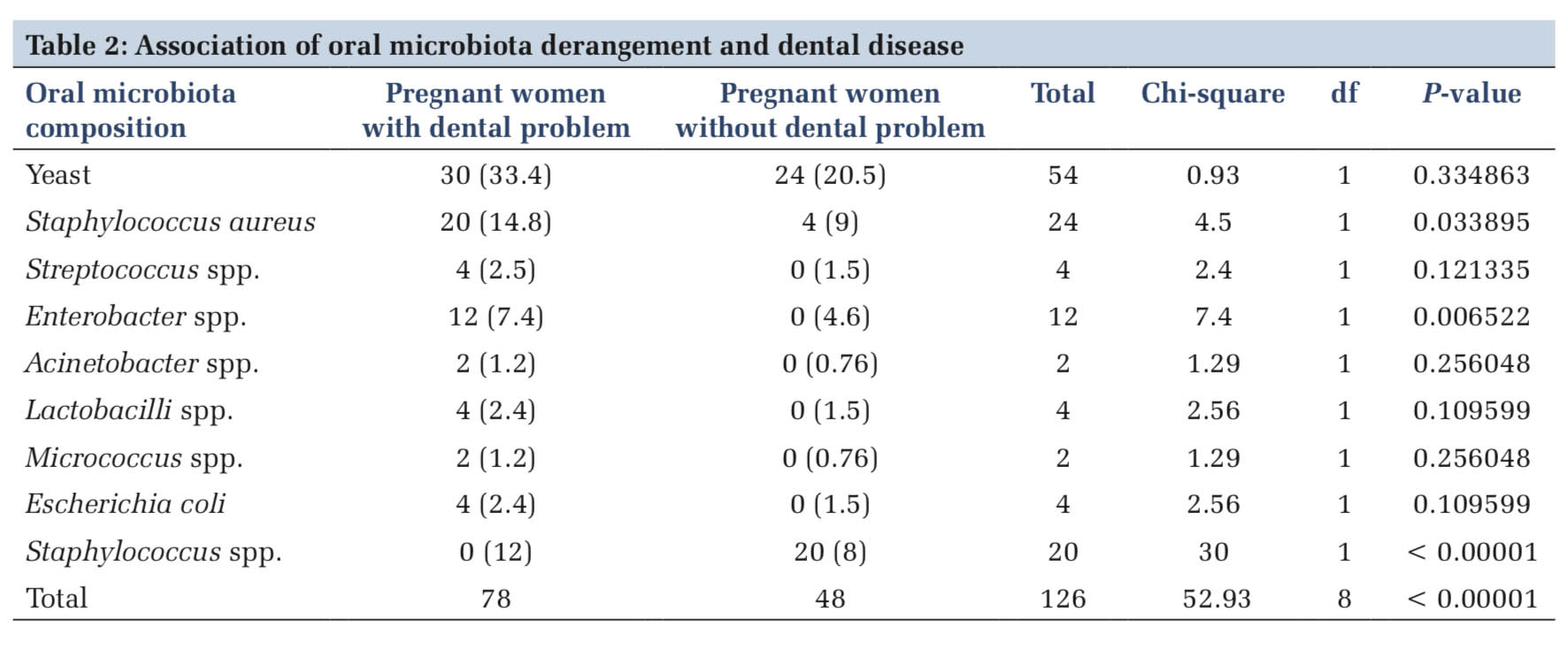
Association of oral microbial community derangement and dental disorders The change of microbiota was analyzed in table. There was statistical significance (x2 =52.93, df = 8, P < 0.00001) for the overall variation of the oral microbiota and dental diseases. For each microorganism, the significant association was observed with S. aureus (x2 = 4.5, df=1, P = 0.033895), Enterobacter (x2 = 7.4, df = 1, P = 0.006522), and Staphylococcus spp. (x2 = 30, df = 1, P < 0.00001). Association was also studied to other oral microbial communities such as Yeast (x2 = 0.93, df = 1, P = 0.334863), Streptococcus (x2 = 2.4, df = 1, P = 0.121335), Acinetobacter (x2 = 1.29, df = 1, P = 0.256048), Lactobacilli (x2 = 2.56, df = 1, P = 0.109599), Micrococcus (x2 = 1.29, df = 1, P = 0.256048), and E. coli (x2 = 2.56, df = 1, P = 0.109599), but there was no statistical significance association was observed. Table2 represents findings regarding the association of oral microbiota, dysbiosis, and dental diseases.
Pregnancy remains the leading cause of oral microbiota dysbiosis among other maternal health states. This study was conducted to analyze the association of oral microbial community dysbiosis and dental diseases among pregnant women. Yeast was the predominant oral microbiota component isolated in both women with dental diseases and those without dental diseases with 100% and 80%, respectively. However, it was high among pregnant women with dental diseases. Staphylococcus spp. were observed among both group but the very low percentage was observed among women without dental diseases with 60% and 13%, respectively. Enterobacter, Acinetobacter, E. coli, Micrococcus, Streptococcus, and Lactobacillus were identified among women with dental diseases, but they were not observed among the control group. Other Staphylococcus spp. were observed among women without dental diseases. Yeast and S. aureus were detected as the most common oral microbial community members among both study groups of women [Figures 1 and 2]. Yeasts are the main components of oral microbiota composition in humans. Yeast was observed to have the highest growth during pregnancy.[11] The current study revealed the high growth of yeasts among pregnant women with and without dental disorders. Some studies reported that the high growth of yeast in the oral route is attributed to hormonal changes that cause a significant reduction of oral PH among pregnant women.[12] The present study did not compare PH level between the studied groups of pregnant women, but the high growth of yeasts was observed compared to other isolated oral microbial communities. Lactobacillus was only isolated among women with dental diseases. Could its presence in the oral cavity influence dental diseases among pregnant women? Previous research revealed that lactobacillus is associated with dental carries among groups of people,[13] although lactobacillus (x2 = 2.76, P = 0.096648) was not statistically significant to be associated with dental diseases regarding findings of the current study [Table 2]. The fact that it was isolated among women with dental diseases clarifies its cooperation with other grown microorganisms to cause dental diseases. S. aureus was isolated among both groups of women studied, but the high prevalence was observed among women with dental diseases, though other Staphylococcus spp. were only isolated among women without dental diseases. The oral cavity harbors S. aureus and other Staphylococcus spp. and mostly found in the mucosal membrane including the oral cavity.[14] The study conducted on Staphylococcus in the oral cavity showed the high significance of the occurrence of Staphylococci in the oral cavity among periodontal health adults.[15] This has no contradiction with the current study where the imbalance on both S. aureus (x2 = 5, df=1, P = 0.025347) and Staphylococcus spp. (x2 = 30, df = 1, P ≤ 0.00001) was statistically significant to be associated with dental diseases among pregnant women [Table 2]. Tooth decay is the most prevalent among dental diseases. Streptococcus is one of the bacteria causing this dental health condition among others.[16] This bacterium was one of 6 microorganisms identified among women with dental diseases, but was not observed among women without dental diseases, however, it was not statistically significant to cause dental diseases. Enterobacter (x2 = 7.4, df = 1, P = 0.006522) was statistically significant to cause dental diseases among pregnant women and was not isolated among women without dental diseases [Table 2]. Enterobacter is from the Enterobacteriaceae family that is opportunistic pathogens of the oral cavity, during pregnancy, these bacteria could cause infections among pregnant women.[17] E. coli, Acinetobacter, and Micrococcus were also isolated among with dental diseases. None of these bacteria was statistically significant, but the fact that they are not significant does not mean their divinity in the oral cavity [Table 2]. Oral microbiota imbalance was observed because there were microorganisms found among women with dental diseases that are not present among women without dental diseases. On the other hand, some bacteria were present in both groups but with unequal percentages. The study conducted on the Bacterial Profile Associated with Dental Caries E. coli was isolated at 7.1% among studied participants.[18] This is a proof that its presence in the oral cavity of pregnant women could lead to dental health conditions. Acinetobacter is non-pathogenic to healthy individuals but it can be harmful in immunosuppression and pregnancy included in the study. Its presence in the oral cavity during pregnancy leads to dental diseases.[19] Despite this condition of Acinetobacter to cause infection in immunosuppressed individual including pregnant women, Acinetobacter (x2 = 1.37, df = 1, P = 0.241812) has not been significant according to the current study [Table 2].


This study was conducted to analyze the association of oral microbial dysbiosis among women with dental diseases. Among the nine oral microbial compositions isolated, only yeasts, S. aureus and Staphylococcus spp. were common between pregnant women with dental diseases and pregnant women without dental diseases. However, only S. aureus, Enterobacter, and Staphylococcus spp. had the significance association with dental diseases among pregnant women. The study recommends that pregnant women should consult a dentist at least once during pregnancy for early detection and treatment or oral health disorders. Prevent oral health disorders, during pregnancy, doctors, nurses, and midwives should provide basic oral health education as part of antenatal care services for optimum oral health among pregnant women.
We acknowledge Gatenga Health Center for sample collection support.
Subscribe now for latest articles and news.