

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2016.v02i03.003
Year: 2016, Volume: 2, Issue: 3, Pages: 13-18
Original Article
D Shobhitha1 , Shameem Shariff2
1Post-graduate Student, Department of Pathology, MVJ Medical College and Research Hospital, Hoskote, Bengaluru, Karnataka, India,
2Professor and Head, Department of Pathology, MVJ Medical College and Research Hospital,
Hoskote, Bengaluru, Karnataka, India
Address for correspondence:
D Shobhitha, Department of Pathology, M V J Medical College and Research Hospital, Hoskote, Bengaluru, Karnataka, India. E-mail: [email protected]
Background: Cysticercosis is a zoonotic disease. It is caused by the larval form of pork tapeworm Taenia solium. This disease is a public health problem. Several developing countries are listed as endemic area for this disease and major among them are countries of Asia, Central Africa, and South America. With the advent of fine needle aspiration cytology (FNAC), attempts have been made with varying success to diagnose these larval forms before definitive excision.
Materials and Methods: This study reports four cases of cysticercosis obtained by FNAC in the Department of Pathology. Furthermore, a complete review of the available Indian literature has been done over the last 38 years for the cytological diagnosis of cysticercosis.
Results: Four cases of cysticercosis were diagnosed at FNAC in the present series. The diagnosis was made on the presence of structure of parasite. A total of 612 cases were diagnosed at cytology in the last 38 years in the English Indian literature. 62.2% of these were diagnosed based on structure of parasite, 12% showed hooklets alone and on the others a perceptive diagnosis of cysticercosis were made.
Conclusion: FNAC diagnosis of cysticercosis can be easily made provided the reporting cytologist is aware of the morphological criteria. Diagnosis can be made as an office procedure before excision of the lesion or institution of the medical line of treatment.
KEY WORDS:Cysticercosis, fine needle aspiration cytology, India
Cysticercosis is a zoonotic disease. It is caused by the larval form of pork tapeworm Taenia solium.[1] The word “Cysticercus” is derived from two Greek words, 'Kystis' meaning cyst and “Kertos” meaning tail due to its appearance.[2]
This disease is a public health problem. Several developing countries are listed as endemic area for this disease and major among them are countries of Asia, Central Africa, and South America.[1] In India, North India is more commonly known for cysticercosis.[3] Due to frequent migration and changes in travel patterns, it is now reported in developed countries also.[1]
The definitive hosts of T. solium are humans, whereas pigs which lodge the larval forms are the intermediate hosts. The common sites of involvement are skeletal muscle, subcutaneous tissue, brain, and eye.[4]
These parasites are usually diagnosed based on their classic morphological appearances on excision of the lesion.[5] They are usually associated with an inflammatory host tissue response.[5] With the advent of fine needle aspiration cytology (FNAC), attempts have been made with varying success to diagnose these larval forms before definitive excision. The diagnostic role of FNAC in cysticercosis was first emphasized by Kung et al., in 1989.[6,7] Presently, diagnosis of these parasitic lesions in various parts of the body using cytomorphological studies is well established.[8]
This study reports four cases of cysticercosis obtained by FNAC in the Department of Pathology.
A complete review of the available Indian literature has been done over the last 38 years to document the occurrence, morphological appearances of the parasite at FNAC, the sites of involvement and the plane of involvement.
Inclusion criteria
1. Cases with cytological diagnosis of cysticercosis retrieved from the archives of pathology
2. Data from Google Scholar, English literature, with cytological reports of cysticercosis in the Indian subcontinent.
Four cases of cysticercosis diagnosed at FNAC were observed in the last 11 years out of 11,131 FNAC
Case 1
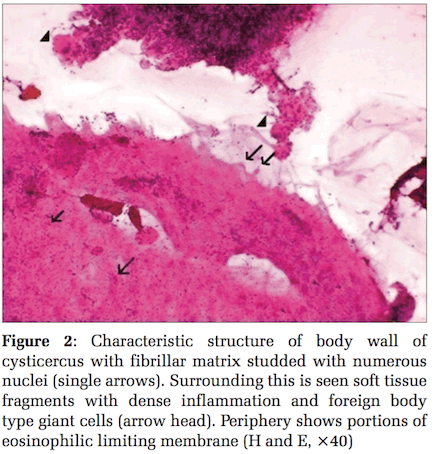
A 30-year-old female, who was a vegetarian by habit, presented with swelling over the anterior abdominal wall, close to the anterior superior iliac spine, measuring 1 cm × 1 cm. This lesion was present in the subcutaneous plane. Fine needle aspiration yielded 0.5 ml of brown aspirate which was smeared onto the slide. Microscopically, it showed fragments of soft tissue infiltrated by eosinophils, histiocytes, plasma cells, lymphocytes, and macrophages. Fragments of laminated eosinophilic membrane morphologically suggestive of the outer limiting membrane of cysticercal larva could be identified (Figure 2). Also seen were 2 hooklets which were refractile on using the ×10 objective. Based on these findings, it was diagnosed as a case of granulomatous inflammation due to cysticercosis.
Case 2
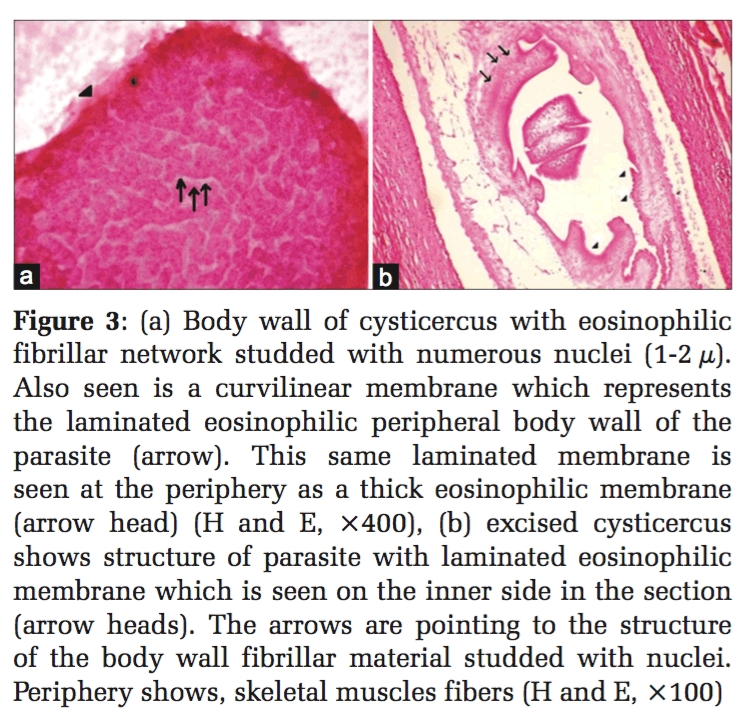
A 25-year-old male, who was a vegetarian by habit, presented with a swelling over the right thigh, measuring 2cm × 0.5cm (in the subcutaneous plane). Fine needle aspiration yielded 0.8 ml of clear fluid. Microscopically, body wall of cysticercus with eosinophilic fibrillar network studded with numerous nuclei (1-2 μ) was identified. Also seen was a curvilinear membrane which represented the laminated eosinophilic peripheral body wall of the parasite (Figure 3a). This same laminated structure is seen at the periphery as a thick eosinophilic membrane. Surrounding this was observed a granulomatous inflammation with histiocytes, lymphocytes, plasma cells, and multinucleated giant cells. Thus, a diagnosis of cysticercosis was made and the lesion excised. It was further confirmed to be cysticercosis on histopathology (Figure 3b).
Case 3
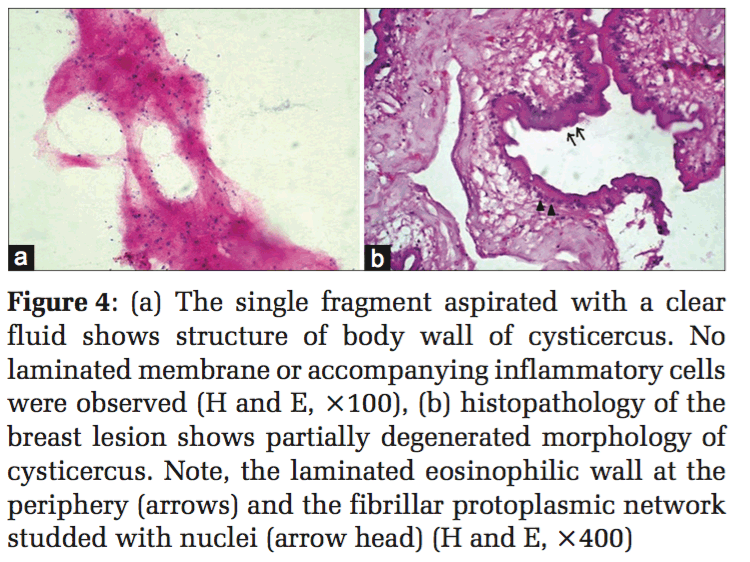
A 40-year-old male, who was a non-vegetarian by habit, with a request for FNAC, presented with a lump in the right breast, insidious in onset measuring 3 cm × 2 cm. The aspirate was clear. On smearing, only a single minute white fragment was identified. This corresponded to the body wall of cysticercosis with fibrillar material and nuclei (Figure 4a). Since the reporting cytologist was familiar with the appearances of the structure of the body wall, the diagnosis of cysticercosis was made on the only available minute fragment. Surgical excision of the lesion confirmed the diagnosis (Figure 4b).
Case 4
A 4-year-old girl, who was a non-vegetarian by habit, presented with a swelling over the right hypochondriac region measuring 3 cm × 2 cm and dorsum of left hand measuring 2 cm × 1 cm since 6 months. The swelling on the dorsum of left hand was aspirated and it yielded a white membranous structure (Figure 6a). Smear showed totally degenerated remnants of parasite with adjacent inflammation (Figure 6b). Based on these findings, it was diagnosed as a case of cysticercosis.
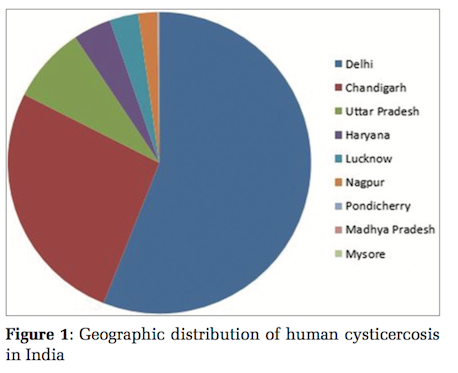
A review of literature showed 612cases of cysticercosis diagnosed at cytology in India, in the last 38 years. These were analyzed. The cases were documented from 1978 to 2013. Majority, i.e., 57% (n = 351) were reported from Delhi,[1,7-15] 25% cases (n = 152) were from Chandigarh,[16,17] 8% (n = 49) were from Uttar Pradesh,[18,19] 4% (n = 22) from Haryana,[3] 2.5% (n = 15) from Lucknow,[20] 1.6% (n = 10) from Nagpur,[21] and 0.2% each (n = 1) from Pondicherry,[2] Madhya Pradesh,[22] and Mysore.[6]
In the review of literature, distribution of the cases was found to be 21.3% of cases (n = 129) from the head and neck region[2,3,9,10,13,14,18,19,21,23,24] and 16.05% cases (n = 97) from the trunk (excluding breast).[3,7,6,9,10,13,23] Head and neck region involved tongue, lips, buccal mucosa, lymph nodes, and rarely orbit. This is followed by the extremities which showed 18.5% cases (n = 109).[3,9,10,12,13,19,23] In the available literature, upper extremity was involved in 8% of the cases (n = 46)[3,10,12,13] and lower extremity was involved in 2.2% (n = 12).[13,19] In 8% of the cases (n = 44), specification of the extremity involved was not mentioned.[24] Breast involvement was seen in 5.7% (n = 35) of the cases.[1,3,8,9,15,19,22] In the remaining 32% cases (n = 188), the site of involvement was not mentioned adequately.[11,16,17,20,21]
The lesion was situated in the subcutaneous plane was seen in 59% cases (n = 349).[6,9,7,11,12,17,19,20,21] Intramuscularly in 1.2% (n = 7) of the cases, whereas visceral cysticercosis was documented in 0.3% cases, i.e., 1 cases alone.[19,21] This was located in the gall bladder. In 39.5% of the cases, the plane of the swelling was not mentioned.[10,13,16,23]
Grossly, clear fluid was aspirated in 30.2% cases (n = 178),[1-3,8,9,11-13,19,23] purulent fluid was aspirated from 10% cases (n = 60),[3,9,10,13,19] and blood mixed aspirate was obtained from 4% cases (n = 23).[10,13] 17% of the cases (n = 105) showed blood mixed purulent aspirate.[9] In the remaining 38.8% cases, the nature of the aspirate was not documented.[14-17,20,21,24,25]
Cytopathologically, 62.2% cases (n = 381) showed the presence of the structure of the parasite.[3,8-11,13-15,17-18,22] The presence of hooklets alone was documented in 12% (n = 72) of the cases (apart from the 62.2% cases with the structure of parasite).[2,9,7,13,19] In 25% of the cases, the diagnosis was made on the presence of host inflammatory response in the cavity wall housing the totally degenerated parasite.[1,3,8,10,11,13,19] Histopathological confirmation was available in 14% (n = 83) of the cases.[1-3,6-8,10,11,13,15,17,21,25]

Cysticercosis is a common infestation worldwide. The prevalence of porcine cysticercosis in India ranges between 7% and 26%.[26] There is no data available regarding its prevalence in the humans in India. The adult worm lodges in the small intestine of humans. The eggs or gravid segments are passed out with feces on the ground. While grazing, the pigs swallow these eggs. In the alimentary canal of the pigs, the radially striated walls of the eggs rupture. This is followed by the release of oncospheres. These oncospheres with the aid of their hooklets penetrate the gut wall. They then make their way to the systemic circulation by breaching the portal vessels or mesenteric lymphatics. Travelling via the portal vein, they successively reach the liver, right side of the heart, lungs, left side of the heart, and systemic circulation. The naked oncospheres get filtered out from the circulating blood and ultimately settle down in the muscular tissues. On consumption of under cooked meat of the intermediate host, humans in turn come to harbor the infestation. Vegetarians are not exempt from this infestation as they may be affected by consumption of contaminated water or uncooked vegetables infected by T. solium eggs or gravid segments of the parasite. The other mode of infection is autoinfection. This occurs by unclean and unhygienic personal habits or by a reversal of peristaltic movements of the intestine. Exvagination in the alimentary canal of humans occurs when the scolex comes in contact with bile. It then anchors to the gut wall using its suckers and develops into an adult worm by gradual strobilization.[4]
Fully developed cysticerci are opalescent milky white cysts (bladders), elongated to oval and about 1 cm in diameter. A typical morphological criterion at histopathology has to be satisfied to diagnose cysticercosis in human tissues - A head with suckers, refractile hooklets, intracorporeal vacuoles, and a thick peripherally located eosinophilic undulating limiting zone with a bilaminate external limiting membrane (Figure 5).[5]
The head is composed of a single invaginated scolex and cyst fluid within the bladder wall. It is this clear fluid which is aspirated at FNAC when the needle enters the bladder wall of the larva. The scolex has rostellum, four suckers, and 22-32 small hooklets.[3] Some authors claim that the scolex can be aspirated in the needle and may be a part of the cytomorphological diagnosis.[15] Parasitic fragments also compose of bluish fibrillary structures, calcospherules, tegument (laminated body wall) thrown into rounded wavy folds.[3]
At cytology, the diagnosis has been made in literature on the presence of fragments of bladder wall, hooklets, and structure of parasite.[2,3,7-11,13-15,17-19,22,23] In the 4 cases of cysticercosis in the present series, fragments of eosinophilic laminated external limiting membrane (Figures 3 and 4) body wall structure which includes fibrillar bladder wall with numerous dots (nuclei) (Figure 2) and/or presence of hooklets clinched the diagnosis. In the authors’ opinion, unless parasite structure is identified, a definitive diagnosis of cysticercosis cannot be established. Cytomorphologically, the majority, i.e.,62.2% of the cases reviewed in literature showed the presence of structure of parasite and 12% cases showed hooklets. The rest of the cases were given a diagnosis suggestive of cysticercosis based on the presence of eosinophils, neutrophils, palisading histiocytes, giant cells, and typical granular dirty background. 14% of the cases were histopathologically confirmed. The host response with inflammation, histiocytic, and eosinophilic infiltrate can only be a suggestion with regard to the presence of a parasite. This should not be considered as a definitive indication of cysticercosis as has been the suggested diagnosis in 25% of reviewed literature.[1,3,10-13,19] The authors, however, may seek justification on the grounds that eosinophils and histiocytes lining a cavity are rarely seen in other conditions. Tuberculosis which can give rise to such a cavity is invariably diagnosed on the additional presence of well-established granulomas either at cytology or histology as well as necrosis.[27]
The inflammation surrounding the parasite due to the human host-tissue reaction may result in chronicity, because of which the viable parasite may undergo degeneration by the phagocytic properties of the host granulomatous response.[5] These structural changes at morphology are reflected even at cytology where portions of the parasite aspirated could be viable or undergoing necrosis.
Grossly, in the present case series, clear fluid was aspirated from 2 cases, one case showed brown fluid and the last case showed purulent fluid. In the literature reviewed, at aspiration, clear fluid was found in 30.2% cases, and purulent fluid was documented in 10% of the cases. The undisturbed bladder/bladder with minimal surrounding inflammation usually contains clear fluid. When the bladder wall is overtaken by inflammation, then the inflammatory exudates may contaminate the clear fluid resulting in a purulent aspirate. FNAC procedure may sometimes make the fluid hemorrhagic.
Among the 4 cases reported at our institution, the sites involved were the trunk, extremities, and breast. In literature, cases were found to arise mainly from the head and neck region involving tongue, lips, buccal mucosa, lymph nodes, and rarely orbit, followed by the trunk. This is followed by the extremities. Multiple sites may be involved in the same patient as seen in Case 4 of the present series. Although the swelling in the right hypochondrium was not aspirated, it could be inferred that this may have the same etiology.
The diagnosis of cysticercosis on clinical grounds alone is a difficult task as it may be confused with benign mesenchymal tumors (such as lipoma, neural tumours, fibrous histiocytomas, etc.) and lymphadenitis.[3,23]
In all the 4 cases reported at our institution, the swelling was found to arise from the subcutaneous plane. In literature, the majority of the swelling, i.e., 59% were found to arise from the subcutaneous plane. The larval forms of T. solium, consequent to their random localization in tissues in humans, are logically transported to the body’s more active muscular portions. This contradictory finding in the subcutaneous plane can be explained by the possibility that, the swelling was actually lodged in the firm underlying skeletal muscle and could have bulged from there into the softer overlying subcutaneous tissue.[5]
FNAC thus plays a major role in its diagnosis as it is a low-cost outpatient procedure. It is a useful tool for pre-operative diagnosis and in some cases and it may even obviate the need for open biopsy as a medical line of treatment (praziquantel and albendazole) is available to deal with this.[3]
In the literature reviewed, the majority of the cases are reported from North India, where this infection appears to be endemic (Figure 1).[1,3,7-14,16-18,20,25] South India, where the present cases were detected, shows sporadic case reports.
After the extensive review of literature, it is found that FNAC diagnosis of cysticercosis can be easily made and provided the morphological criteria is adequately met with the viable or degenerating parasite. In other cases, a suggestive diagnosis is warranted. This paper emphasizes the need for cytopathologists to be aware of the structure of cysticercus in aspirates, which would enable an easy diagnosis. A soft tissue diagnosis of cysticercosis can aid the patient in averting life-threatening complications of neural cysticercosis.
Subscribe now for latest articles and news.