

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2016.v02i03.004
Year: 2016, Volume: 2, Issue: 3, Pages: 19-24
Original Article
K Vanitha1 , N Sasivathanam2 , K Nirmala Devi3 , M Ashok4 , N Santhi1
1Assistant Professor, Department of Biochemistry, K.A.P.V. Government Medical College, Trichy, Tamil Nadu, India,
2Professor and Head, Department of Biochemistry, Thanjavur Medical College, Thanjavur, Tamil Nadu, India,
3Professor and Head, Department of Biochemistry, K.A.P.V. Government Medical College, Trichy, Tamil Nadu, India,
4Assistant Professor, Department of Medicine, K.A.P.V. Government Medical College, Trichy, Tamil Nadu, India
Address for correspondence:
Dr. K Vanitha, Assistant Professor, Department of Biochemistry, K.A.P.V. Government Medical College, Trichy, Tamil Nadu, India. Phone: +91-04312512480, 9842514543. E-mail: [email protected]
Background: Acute coronary syndrome (ACS) denotes the acute phase of ischemic coronary artery disease with or without myocardial necrosis. It is the initial working diagnosis later refined by electrocardiography and biomarkers. Diagnostic sensitivity and specificity for ACS, especially at the earliest stage remains insufficient. Pentraxin 3 (PTX3) is the inflammatory protein and has the potential to be a biomarker in the diagnosis of ACS, as it is elevated in the early hours (6-8 h) of ACS. The aim of the study is to estimate serum PTX3 level in patients with ACS and to correlate with serum creatine kinase-MB (CK-MB) and lipid parameters. Materials and Methods: A study group includes 50 patients who were admitted in Intensive Cardiac Care Unit with ACS and 50 participants of age- and sex-matched healthy individuals were taken as control. Assay of serum PTX3 was done by ELISA method and CK-MB by kinetic immune inhibition method. Serum lipid profile was measured by colorimetric method. Results: Serum PTX3 levels (mean: 5.607 ± 2.82 ng/ml) were elevated in the ACS compared to normal healthy persons (mean: 1.38 ± 0.52 ng/ml) and are statistically significant (P < 0.05), and there is no correlation between serum PTX3 levels, CK-MB, and lipid profile. Conclusion: The elevated concentration of serum PTX3, in early hours (6-8 h) of ACS, is a novel biomarker for diagnosing patients.
KEY WORDS:Acute coronary syndrome, creatine kinase-MB, lipid parameters, pentraxin 3
Cardiovascular disease (CVD) is the largest cause of morbidity and mortality in India. The global status on noncommunicable disease report 2011 says more than 2.5 million deaths are from CVD, of which 2/3rd is due to coronary heart disease (CHD).[1] CHD is assuming serious dimensions in developing countries. It is the most important cause of death in India, in the year 2015.[2] By 2020, one in every three deaths will be due to CVD worldwide.[3] The term acute coronary syndrome (ACS) denotes the acute phase of ischemic coronary artery disease later refined by electrocardiography (ECG) and biomarker. Onset of ACS involves rupture or erosion of atherosclerotic plaques in coronary arteries.[4] In the emergency department, despite the clinical experience of emergency care practitioners, evaluating the patient with chest pain is a challenging task. In spite the use of ECG and biochemical parameters, 2-5% of ACS patients are inappropriately discharged.[5] Now, the cardiac biomarkers such as troponin T or I and creatine kinase-MB (CK-MB) are clinically utilized to diagnose ACS diagnostic sensitivity and specificity of those for ACS, especially at the earliest stage remain insufficient. Inflammation participates in the atherosclerotic process from the beginning till end[6] that is in the initiation, development, and progression of atherosclerosis.[7] Inflammatory biomarkers are useful not only for diagnosis but also as indicators of disease trait (risk factor or risk marker), disease state (preclinical or clinical), or disease progression or prognosis.[8] The association of increased serum levels of acute phase proteins with the progression of atherosclerosis and with the occurrence of atherosclerosis-related adverse events such as ACS have been well documented in several epidemiological studies.
This age-and sex-matched cross-sectional study was conducted at Thanjavur Medical College Hospital after getting approval from the Ethical Committee and written informed consent from the participants. The study group includes 50 patients aged 30-65years, admitted with complaints of chest pain within 6-8 h of onset, chest pain lasting >30 min, and clinical findings suggestive of non-ST- segment elevation MI (NSTEMI) (n = 8), unstable angina (n = 10), and STEMI (n = 32). The exclusion criteria considered in the study population were a stroke, symptomatic peripheral artery disease, infection, vasculitis, patients who have undergone angioplasty, bypass surgery, or open heart surgery, chronic kidney disease, cardiac disease other than coronary disease, rheumatoid arthritis. 50 sex- and age-matched healthy individuals were taken as controls. Venous blood from the control and study groups was collected by venipuncture with strict aseptic precaution (as soon as the patients got admitted as per the inclusion criteria). All the blood samples were centrifuged at 3000 rpm for 10 min and serum was separated. One part of the serum sample was taken for analysis of CK-MB. The remaining part of the serum sample was stored for analysis of PTX3 at −20°C. Fasting blood samples were also collected from all participants during their hospital stay and analysis of total cholesterol (TC), triglycerides (TGL), and high-density lipoprotein cholesterol (HDL-C) were done.
Serum PTX3
Serum PTX3 level was estimated by human serum PTX3 ELISA kit - (Catalog No. SK00101-01) Aviscera Bioscience Inc., California.
Principle of the assay
This assay employs the quantitative sandwich enzyme immunoassay technique. An antibody specific for PTX3 has been precoated onto a microplate. Standards and samples are pipetted into the wells, and any PTX3 present is bound by the immobilized antibody. After washing away any unbound substances, a biotinylated antibody specific for PTX3 is added to the wells. Following a wash to remove any unbound antibody-biotin reagent, horseradish peroxidase link streptavidin is added to the wells. After washing away any unbound enzyme, a substrate solution is added to the wells and color develops in proportion to the amount of PTX3 bound in the initial step. The color development is stopped, and the intensity of the color is measured. The range of the assay was between 0 and 14 ng/ml and the expected value for the healthy individual was in men 1.81-1.94 ng/ml and in women 2.05- 2.19 ng/ml. Analytical sensitivity of this kit was 0.02 ng/mL. The intra assay precision was 4-6%, and inter assay precision was 8-10%.
Serum CK-MB measured by kinetic immune inhibition method. Serum TC was estimated by cholesterol oxidase-peroxidase method, serum TGLs by glycerol phosphate oxidase-phenol aminophenazone method, and serum HDL by direct method. Calculated parameters were serum HDL and serum very low-density lipoprotein (VLDL) using formula: LDL-C = TC-[HDL+TGL/5]; VLDL-C = TGL/5.
Statistical analysis Student’s t-test was employed for the statistical analysis of data. The data were expressed in terms of mean and standard deviation. P < 0.05 was taken as significant value. Correlation between the measured parameters was assessed using Pearson’s coefficient of correlation using SPSS software.
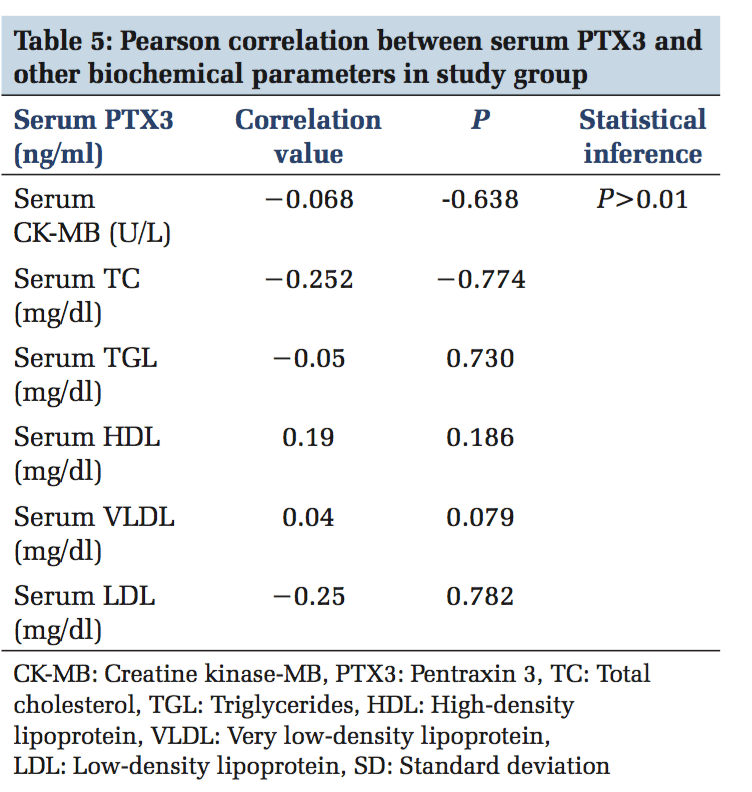
A total of 100 participants were included in this study. Patients with ACS (n = 50) and healthy individuals (n = 50) were under the control group. Both in the study and control group males (n = 36) and females (n = 14) were included. The serum levels of PTX3 and serum CK-MB, lipid parameters levels were estimated in control and study group. In Table 1, serum PTX3 levels with a mean of 1.3826 in the control group, and in the study group, serum PTX3 levels with a mean of 5.6074 which is higher than the mean of the control group, and the P value is significant. In Table 2, serum CK-MB in the control group with a mean of 13.88 U/L, and in the study group, serum CK-MB in the control group with a mean of 52.96 U/L is higher than the control group mean level, and the P value is significant. Table 3 shows lipid parameters in control and study group, and there is a statistical elevation of serum TC, serum TGL, serum VLDL, and serum LDL-C in the study group compared to control (P = 0.000 < 0.05). There is statistically significant decrease in the serum HDL-C levels in the study group compared to control (P = 0.000 < 0.05). Table 4 is the frequency table which compares the duration of onset of ACS and the number of patients having elevated levels of serum PTX3 and CK-MB. Table 5 shows Pearson correlation between serum PTX3 with CK-MB, TC, TGL, LDL, and HDL. In Figure 1 receiver operating characteristic (ROC), the area under the curve for PTX3 is 0.970, and the area under the curve for CK-MB is 0.923.
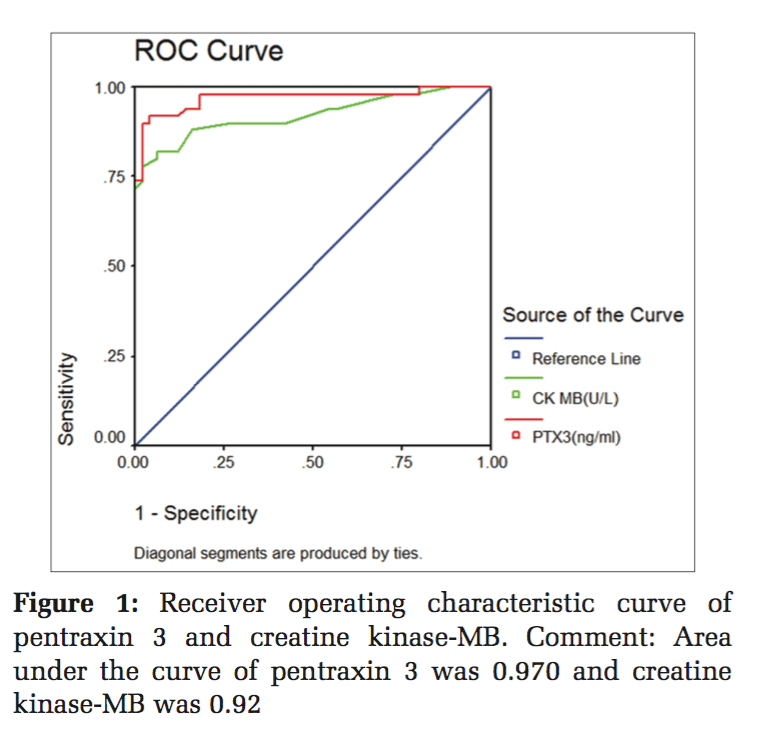
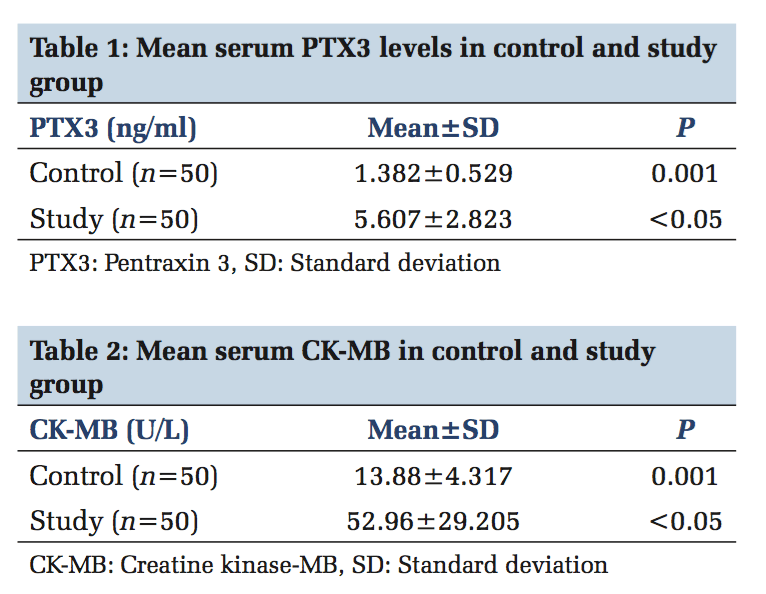
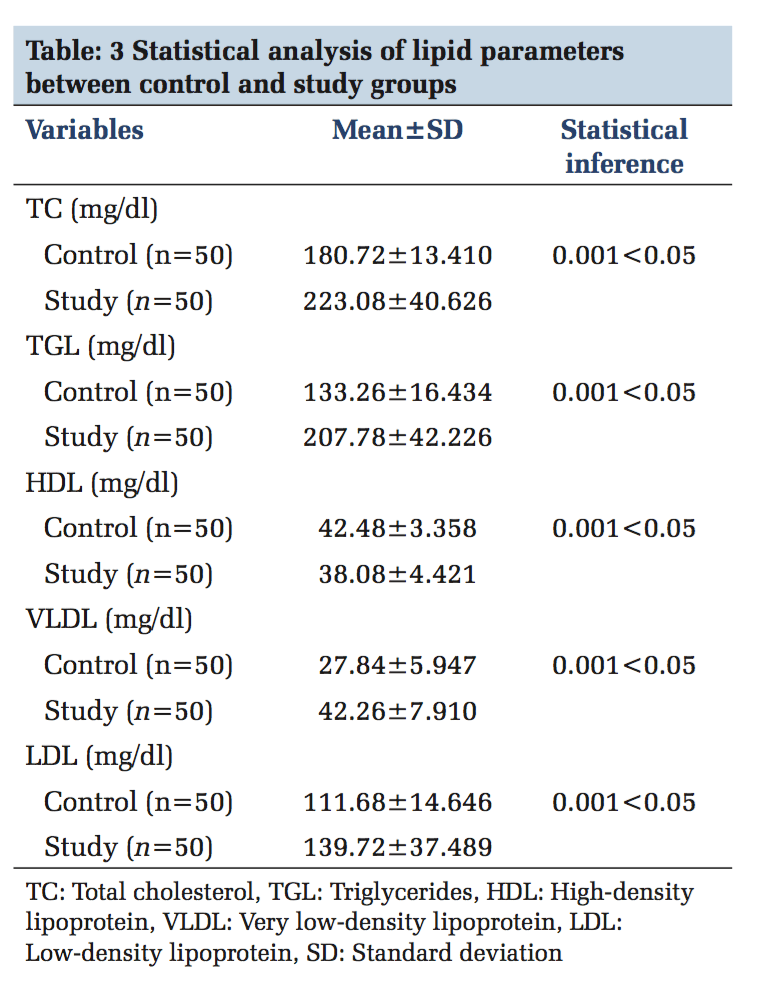
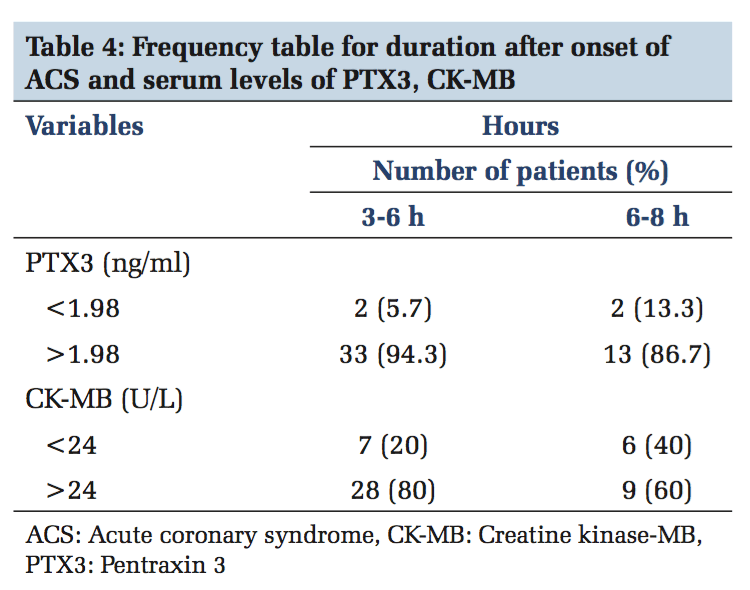
Inflammation is a critical component of ischemic HD (IHD). It is well documented that immunological and inflammatory process play a fundamental role in atherogenesis. CRP is the known member involved in atherosclerosis. Recently, PTX3 has moved into the focus of research.[18] PTX3 is a multifunctional acute phase protein with their roles that are only now beginning to be unraveled. Proinflammatory and oxidative stress appear to play key roles in the formation of erosion and rupture-prone vulnerable atheromatous plaques, by apoptosis of vascular endothelial and smooth muscle cells and macrophages, production and activation of matrix metalloproteinases as well as induced expression of chemokines, and endothelial leukocyte adhesion molecules. PTX3 is also said to be involved in plaques vulnerability.[19] In the present study, mean serum PTX3 of study group (5.60±2.82) is higher than the control group, which is statistically significant (P < 0.05) and this rise in serum PTX3 level is mainly due to the neutrophils which undergo activation across the coronary vascular bed in patients with ACS. Neutrophils represent a source of the PTX3 in blood of patients undergoing AMI, and it was found that intracellular PTX3 is indeed depleted during AMI.[20] This is an acute and transient event, which parallels the early rise in plasma concentration of it within 6-8 h of AMI, and it is proved in previous studies by staining neutrophils with CD15 antibodies.[21] PTX3 is present in neutrophils as secondary granules or specific granule. Activation of the neutrophils across the coronary vascular bed which is the hallmark of ACS is the cause for elevated concentration of serum PTX3 in STEMI, NSTEMI, and unstable angina.[22] The PTX3 level peaks at 7.5 h following AMI and returns the baseline within 3-5 days. Noriakikume et al. have compared the diagnostic values of PTX3 for ACS (in the first 6 h) with other biomarkers of myocardial damage such as cardiac troponin T (CTnT) and heart-type fatty acid binding protein.[23] Another study concluded that PTX3 is a sensitive and specific marker when compared with cardiac markers such as neutrophil activating peptide 2, CTnI within the first 6 h of onset of chest pain.[24] In case of unstable angina, Inoue et al., in their studies, found that the plasma PTX3 levels were three times higher than the normal individuals.[25] Koga et al. performed the optical coherence tomography to assess whether the plasma PTX3 can be a predictor of thin cap fibroatheroma.[26] In that PTX3 level was significantly elevated with thin cap fibroatheroma and suggest that plasma PTX may be a useful biomarker in reflecting plaque vulnerability. The present study mean serum TC level is higher than the control group (223.08 ± 40.62 vs. 180.72 ± 13.41), which is statistically significant (P < 0.05). The elevated serum TC level of more than 150 mg/dl is the risk factor for IHD which was shown in previous studies. The mean serum TGL in the study group (207.78 ± 42.22) which is more elevated than in the control group (133.26 ± 16.43) which is statistically significant (P < 0.05). The incidence of CAD is doubled, if serum TGL increases from 90 mg/dl to 180 mg/dl. In the present study, the mean serum LDL-C of the study group is (139 ± 37) higher than the control mean (111 ± 14) which is significant statistically. Elevated LDL-C has been recognized as a primary risk factor for CAD. The mean serum HDL-C which is lower in the study group compared to the control group (38.08 ± 4.42 vs. 42.48 ± 3.35) which is statistically significant (P < 0.05). As per recommendation of the National Cholesterol Education Program adult treatment panel III risk classification for HDL-C levels the serum HDL-C level < 40 mg/dl is considered as high risk for IHD. Frequency table shows 94.3% increase of PTX3 in the early (3-6) h, whereas CK-MB has 80% and the ROC curve denotes the area under curve for PTX3 is more than CK-MB. This clearly shows that serum PTX3 can be used as a novel marker in ACS patients.
No statistically significant positive or negative correlation was found between PTX3 with CK-MB and lipid parameters (P > 0.01). So, serum PTX3 is an independent biomarker of ACS.
In this study, the elevated level of serum PTX3 in the early hours (3-6 h), clearly shows that it can be used as a novel marker in the diagnosis of ACS. PTX3 being an immuno-inflammatory marker, its estimation in the early hours of ACS aids in the diagnosis and management of ACS.
Subscribe now for latest articles and news.