Introduction
Ameloblastoma is a rare odontogenic tumor of jaw accounting for 1% of all cysts and tumors of the jaws, more so the acanthomatous variant.[1] Although histopathology and imaging findings of this tumor have been well documented, fine-needle aspiration cytology (FNAC) reports are rare.[1-4]
Clinically and radiologically ameloblastoma mimics various odontogenic tumors and cysts as a result a pre-operative accurate diagnosis is indispensable in planning appropriate treatment. At cytology, the predominance of basaloid cells in ameloblastoma brings plethora of lesions, especially salivary gland tumors into the differential diagnosis.[5]
Among several factors which determine the recurrence rate of ameloblastoma, early and accurate diagnosis plays a major role as it is related directly to the adequacy of excision.[6] Although incisional biopsy is usually preferred in oral and jaw lesions, it fails if the needle hits a cystic area since biopsy is usually done at one site to prevent trauma and bleeding.[7] However, FNAC has an advantage of multiple sampling and if cystic area is encountered, the fluid can be examined after sedimentation. Despite this FNAC is not routinely used as adjunct tool in diagnosis of odontogenic tumors, especially ameloblastoma, the reason being lack of specific diagnostic criteria. Cytology becomes more challenging when it camouflages as a parotid tumor.
We describe a rare case of acanthomatous variant of ameloblastoma diagnosed at cytology.
Materials and Methods
A 28-year-old male, a diagnosed case of schizophrenia, was referred to cytology section with a parotid swelling, initially diagnosed as pleomorphic adenoma 1 year back. Based on the location and the previous FNAC report which was done outside, a provisional clinical diagnosis of pleomorphic adenoma was made. He complaint of trismus on the left side. Swelling was 5 cm × 5 cm present over the left body of mandible extending up to the left parotid region.
It was non-tender and firm in consistency. Skin above the swelling was normal. There was no ulceration of signs of inflammation present over the swelling. The swelling was fixed and immobile. There was no evidence of regional lymphadenopathy. Biochemical and hematological investigations were within normal limits. General systemic examination and other relevant investigations did not reveal any metastasis.
Computed tomography scan showed multilocular radiolucent lesion involving left angle and ramus of the mandible favoring a neoplastic etiology. FNAC was done in the outpatient department without any local anesthesia. A 23-gauge needle with 10 cc syringe as used. Aspiration was done from multiple sites and smears were fixed in 50% ethyl alcohol and air dried as well. Hematoxylin and eosin stain and May Grunwald Giemsa stain were performed.
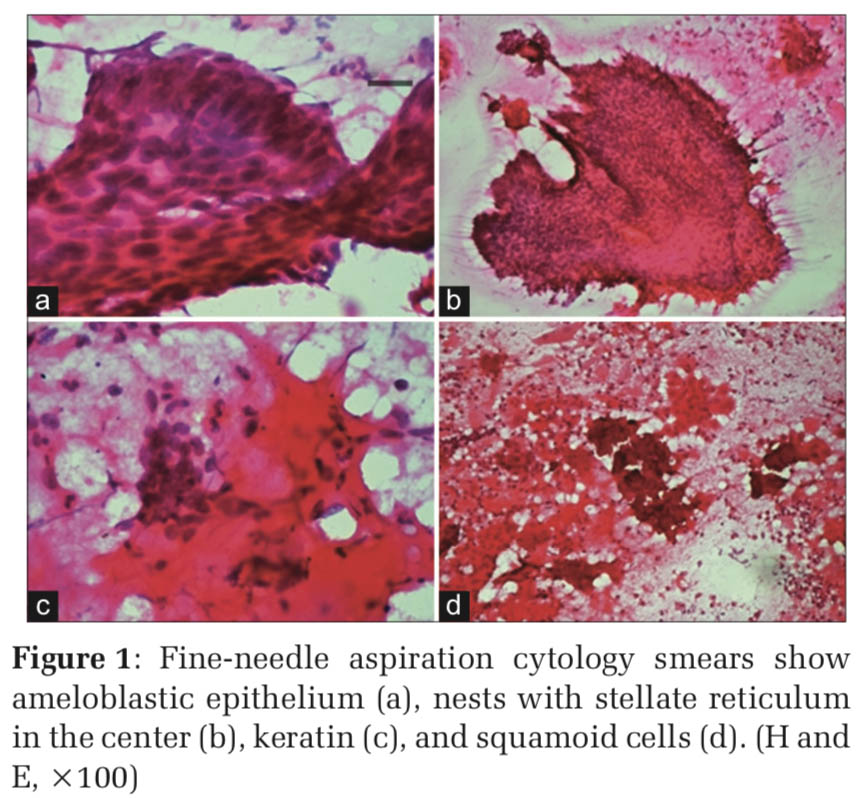
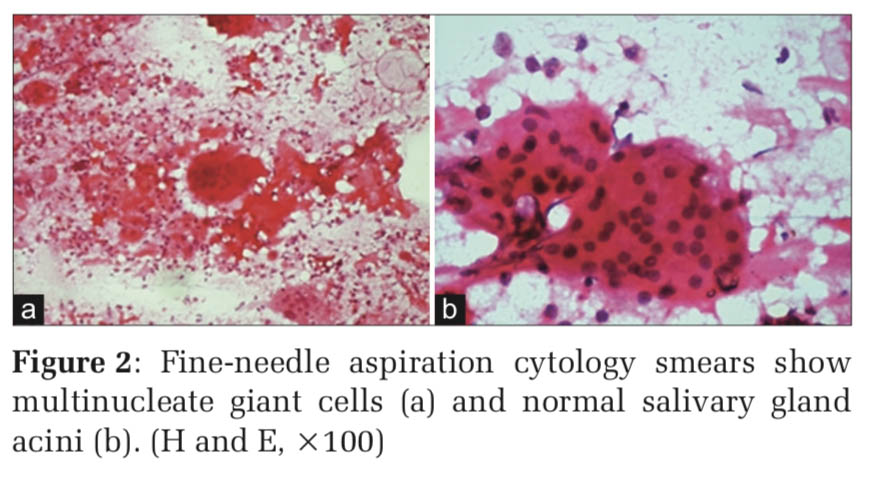
Microscopy Smear study showed microbiopsies, cohesive sheets, pseudopapillary pattern, and solid nests of basaloid cells with peripheral palisading along with many multinucleated giant cells and keratinous material. The basaloid cells had bland chromatin, scanty cytoplasm.
At places, squamoid cells were seen adjacent to the basaloid cell clusters. Spindle-shaped cells in small clusters, monocytoid cells, and normal clusters of salivary gland acini were also noted (Figures 1 and 2).
There was no evidence of atypia, mitosis, or necrosis noted in smears examined. A final diagnosis of acathomatous ameloblastoma was made. A radical surgery was performed. Gross: Specimen consisted of mandibulectomy specimen with a tumor mass measuring 4 cm × 5 cm. Cut surface was solid with whitish friable appearance. There was evidence of hemorrhage and necrosis.
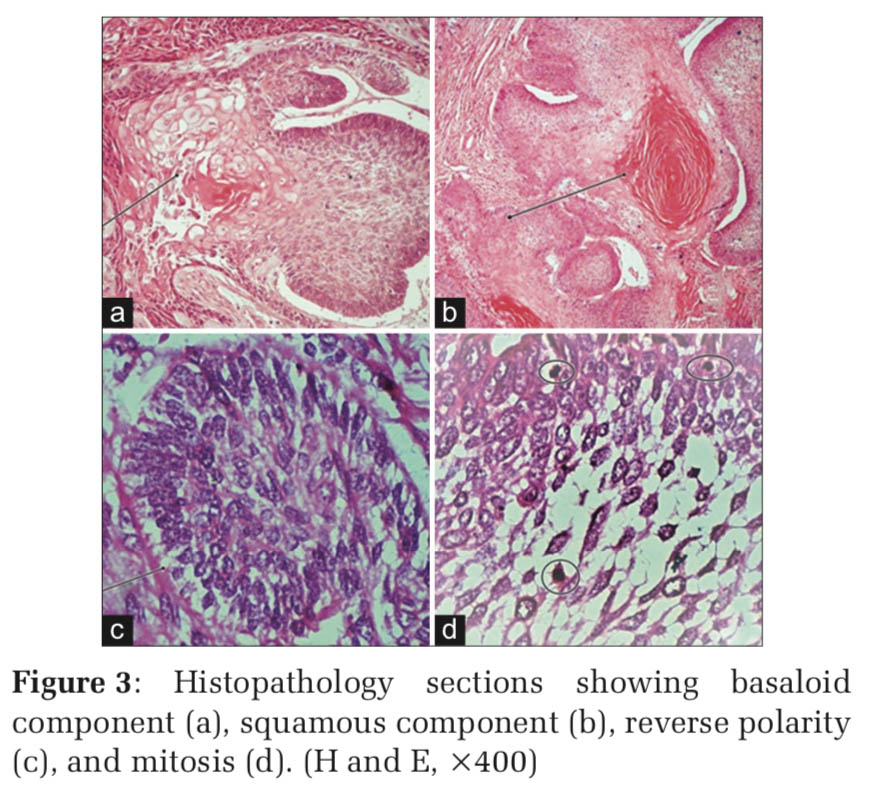
Histopathology revealed solid epithelial cell nests with peripheral palisading and central stellate reticulum. Many cell nests also showed squamous differentiation with well-formed pearls. Areas of mitosis >5/10 High power examination, pleomorphism, and focal necrosis were noted (Figure 3).
Hence, a final diagnosis of acanthomatous variant of ameloblastic carcinoma was made.
Discussion
An incidence of ameloblastoma of 0.5 new cases per million people makes it a rare disease.[8] Furthermore, it is the most common odontogenic tumor of epithelial origin, accounting to 1% of tumors of cysts and jaws.[6] Ameloblastomas arise from ameloblasts, the epithelium involved in enamel formation. Ameloblasts are elongated, columnar cells with reverse polarization and are involved in enamel formation. Despite this, ameloblastomas by definition do not produce enamel.
Ameloblastoma is a locally aggressive neoplasm of low malignant potential. There are four distinct types of ameloblastoma each characterized by unique clinical, radiological, and histological findings. Identification of each type is important in view of the varying clinical behavior and treatment protocol.[6] Although metastatic potential of ameloblastoma is low, the recurrence rate is quite high depending on the mode of surgical treatment adopted.
Conservative surgical approach such as enucleation and curettage results inahighrecurrencerateof55–90%.[6]Incontrast, a radical treatment with osteotomy with adjuvant therapy leads to a low recurrence rate of 15–25%. However, a patient undergoing radical treatment has to face esthetic and functional impairment and also the brunt of reconstructive surgery. In view of this, a presumptive pre-operative tissue diagnosis is important for selecting appropriate treatment.
Various techniques utilized for diagnosis of ameloblastoma include incisional biopsy, FNAC, excision biopsy, the lesser invasive one’s being FNAC, and incisional biopsy. Due to inherent nature of ameloblastoma, it being solid and cystic, incisional biopsy has limitations with respect to sampling error, especially when the needle hits cystic area, thereby leading to misinterpretation.[9]
FNAC is a simple, easy, rapid, cost-effective tool in initial diagnosis of superficial palpable lesions with a high diagnostic accuracy. In head-and-neck lesions, the utility of FNAC is well established. In oral and jaw lesions, the potential of FNAC has not been explored much with variable outcome. Few studies have revealed the importance of using FNAC as first line investigation in intraosseous lesion of oral and maxillofacial region.[10]
FNAC has an important role to play in pre-surgical diagnosis of ameloblastoma to plan adequate treatment, thereby reducing recurrence. FNAC does not require local anesthesia. Multiple sampling can be performed in one setting. Rapid interpretation of material aspirated may provide direction for next plan of action. The only limitation is when the needle hits the cystic area. However, at such time, the fluid can be aspirated and the sediment smears from the fluid may provide valuable information. Similarly, FNAC can be repeated from the solid area, with multiple sampling increasing the accuracy of the test. Cortical bone overlying ameloblastoma often undergoes thinning and at time produces a breach which allows easy penetration of needle. Studies reported so far on cytology of ameloblastoma did not reveal any adverse effects or complications due to FNA procedure.
Despite these advantages, FNAC has not been used as a choice of test for diagnosis of odontogenic neoplasms more so for ameloblastoma. The reason being the lack of specific diagnostic criteria due to scarcity of literature and the easy accessibility of the lesion, paving way for incisional biopsy. Another major limitation of FNAC was the insufficient material obtained due to cystic degeneration. Nevertheless, as mentioned earlier, the choice of site for FNAC along with evaluation of sediment smears would help reduce this limitation.
Ucok in a study of 39 cases of ameloblastoma obtained insufficient material in only one case. Similarly, Gunhan in a study comprising 10 cases could obtain diagnostic material in all the cases.[11] August et al. obtained insufficient material in 2 out of 32 cases of intraosseous jaw lesions.[12] Proper positioning of needle, proper fixation of lesion, aspiration from multiple sites, and efficiently negotiating needle in all directions will help reducing the rate of insufficient material.
Smears of ameloblastoma are characterized by basaloid cells with peripheral palisading akin to ameloblasts, spindle cells representing stellate reticulum, and squamous cells. Radhika et al. in a case series of three histologically confirmed cases of ameloblastoma mentioned the presence of basaloid cells in tight clusters and squamous cells in all the cases.[13] Mathew et al. described the presence of basaloid cells, squamous cells, and spindle cells in their case report.[3] Out of 42 cases of oral and jaw lesions, an accurate diagnosis of ameloblastoma was rendered by Goyal et al. in all the 3 cases with a sensitivity and specificity of 100% each.[10] Diagnosis is not difficult when characteristic combination of these elements appears in the smear, in association with clinicoradiological features. However, in our case, since the lesion was located in the parotid region and previous FNAC report, which was done outside, together leads the clinician to a provisional diagnosis of pleomorphic adenoma. Nevertheless, the basaloid cell population and the location of tumor in parotid region may redirect the cytopathologist to consider various basaloid neoplasms in differential diagnosis which include basal cell adenoma, cellular pleomorphic adenoma, adenoid cystic carcinoma, and adnexal neoplasm. Basal cell adenoma is uncommon in parotid gland. Although palisading may be seen in basaloid cells of basal cell adenoma, the cells are distinctly low cuboidal as compared to tall columnar cells of ameloblastoma. Similarly, the basement membrane material and hyaline globules seen in basal cell adenoma are altogether absent in ameloblastoma. Cellular pleomorphic adenoma may mimic ameloblastoma; however, careful search for chondromyxoid fibrillary stroma and plasmacytoid cells will resolve the issue. Adenoid cystic carcinoma also shows basaloid cells which, however, differs from the cells seen in ameloblastoma by displaying hyperchromasia and nuclear molding. Another distinctive feature of adenoid cystic carcinoma is the presence of hyaline stroma forming hyaline globules and cup-shaped structures formed by basaloid cells. Squamous cells are never seen in adenoid cystic carcinoma.[14] At times, adnexal tumors arising in the dermis may pose diagnostic challenge as these usually demonstrate basaloid cells and nuclear palisading. Malignancy in ameloblastoma is characterized by cytologic atypia, necrosis, and mitosis. There are very few case reports of ameloblastic carcinoma diagnosed at cytology.[5]
Other intraosseous tumors such as mucoepidermoid carcinoma, lymphoma, small cell carcinoma, squamous cell carcinoma, and soft-tissue sarcomas are all look-alikes of ameloblastoma at cytology in view of the presence of basaloid cells in each of them. In lymphoma, the tumor cells are always arranged in a dispersed in contrast to tight clusters of basaloid cells in ameloblastoma. The background in smears of lymphoma shows distinct lymphoglandular bodies, a noteworthy hint not to be missed. In mucoepidermoid carcinoma (MEC), the predominance of intermediate cells may bear a resemblance to basaloid cells of ameloblastoma; however, a careful search should be made for the presence of clear cells and mucous cells whose presence favor a diagnosis of MEC.
One out of 13 cases of ameloblastoma diagnosed a diagnosis of acanthomatous variant was rendered at cytology which was confirmed at histopathology.
In limited number of case series reported in the literature, the sensitivity and specificity of FNAC in diagnosis of ameloblastoma was 86.6%–100% and 100%, respectively.[7] Chandavarkar et al. in a study of 12 cases of ameloblastoma could provide an accurate diagnosis in only 7 cases with a sensitivity of 58.3%.[4]
|

