

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2017.v03i03.001
Year: 2017, Volume: 3, Issue: 3, Pages: 1-12
Review Article
Deepa Bangalore Gotur
Department of Medicine, Weill Cornell Medical College, Intensivist, Houston Methodist Hospital, Houston, TX, USA
Address for correspondence:
Deepa Bangalore Gotur, Department of Medicine, Houston Methodist Hospital, 6565 Fannin, Houston, TX, USA. E-mail: [email protected]
Sepsis has a new definition, and it is defined as dysregulated host response and organ dysfunction due to infection. To clearly define and screen for organ dysfunction, sequential organ failure assessment (SOFA), and quick SOFA scoring system is recommended. Septic shock is a subset of sepsis in which profound circulatory, cellular and metabolic abnormalities are associated with a higher mortality risk. Sepsis incidence in India is under-reported. Inflammatory process and coagulation are closely linked in sepsis pathogenesis. Lactate measurement and its clearance are used both as a diagnosis and management tool for resuscitation in sepsis. Major recommendations by surviving sepsis campaign (SSC) for the management of sepsis are grouped in bundles of interventions. Recognition of golden hour in sepsis for early antibiotics and resuscitation is crucial. 30 cc/Kg crystalloid fluid bolus for septic shock should be infused within 3 h of triage or sepsis diagnosis. Fluid resuscitation in septic shock can be described in four stages - the rescue, optimization, stabilization, and evacuation phases. Instead of targeting distinct values of central venous pressure and mixed venous oxygen saturation, the SSC guidelines now recommend to re-assess volume status and tissue perfusion within 6-h by repeated focused exam and lactate clearance. The first line vasopressor recommended in septic shock is norepinephrine. For patients with sepsis-induced adult respiratory distress syndrome, using higher over lower positive end-expiratory pressure, lower over higher tidal volume setting on the mechanical ventilator, and prone positioning is recommended. A protocolized approach should be used for blood glucose management in patients with sepsis, commencing insulin dosing when two consecutive blood glucose levels are >180 mg/dL and maintaining upper blood glucose level ≤180 mg/dL rather than ≤110 mg/dL. Assessment of nutritional status using scoring systems such as NUTRIC score and NRS 2002 should be made, and early enteral trophic feeding should be initiated and advanced within 24–48 h. Any initiative designed to improve adherence to the sepsis guidelines and thus improve performance in sepsis core measures requires an institution-specific, strategic, and planned approach. A trans-disciplinary team charged with the functions of raising sepsis awareness, developing sepsis focused educational programs, establishing a care pathway model and monitoring compliance and adherence to the sepsis bundles can help improve the sepsis outcomes. Future focus in sepsis is on earlier recognition, newer screening tools, education among public and health-care workers and optimizing recovery.
KEY WORDS:Sepsis, septic shock, SOFA, qSOFA, lactate, sepsis bundles.
Sepsis is defined as the dysregulated host response and organ dysfunction due to infection. The term sepsis comes from a Greek work called sipsi which mean “make rotten.” First ever sepsis research was designed by the famous Hungarian obstetrician Ignaz Semmelweis (1818–1865), who duly observed that the prevalence of puerperal sepsis was higher in women who were delivered in hospitals compared to those who gave birth at home attended by midwives. He proposed that this was due to the contamination of the medical students’ hands as they attended to these deliveries after performing autopsies. He recommended hand washing with a chlorine solution of lime before they would attend to the women in labor and resultantly brought down the mortality rates. Despite this positive trial, his peers considered him a lunatic and was incarcerated, shackled and ironically died from sepsis from pressure wounds, the same disease he had fought for in his entire medical career. Although the concept was known for ages, the first-ever modern-day definition was given by Robert C. Boone in 1989 as “Sepsis is defined as an invasion of microorganisms and/or their toxins into the bloodstream, along with the organism’s reaction against this invasion."
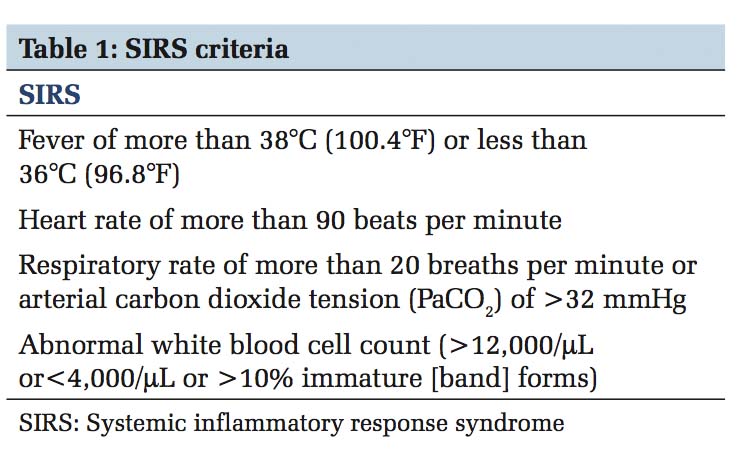
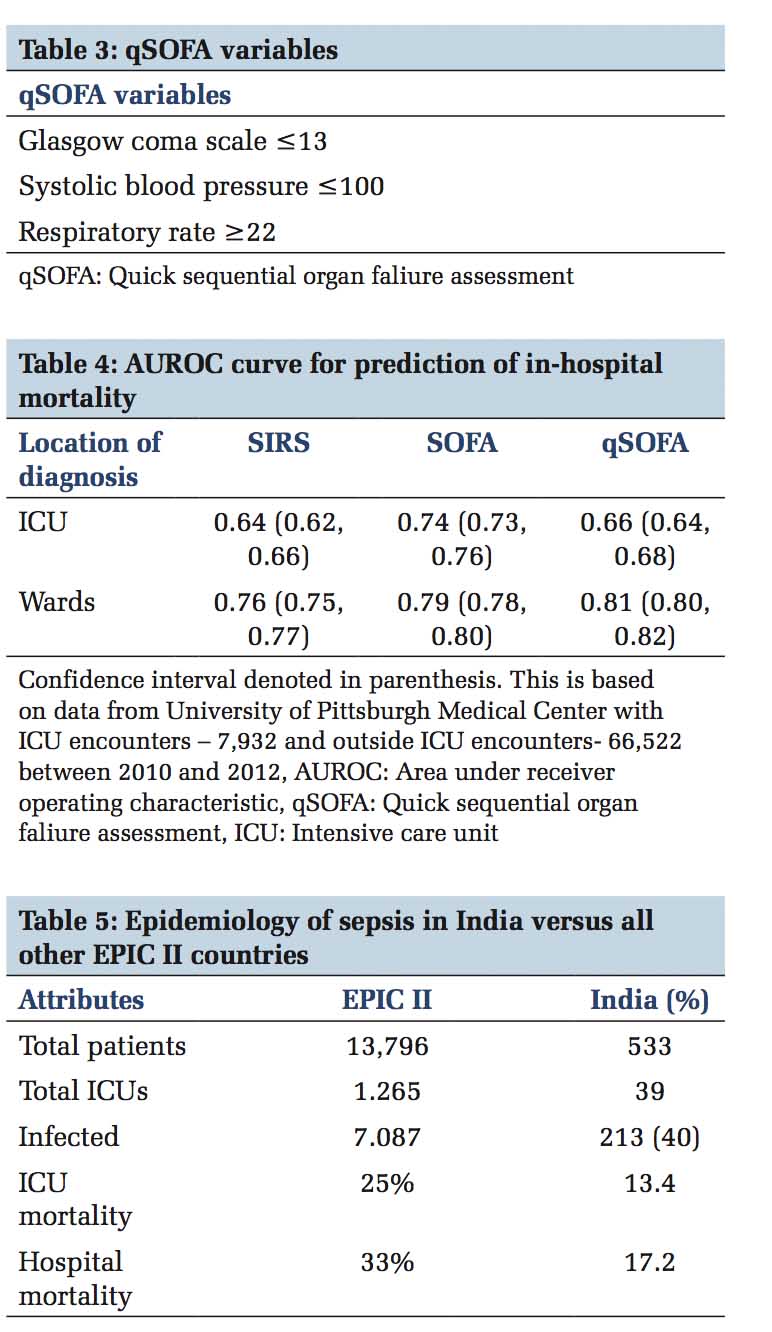
Old definition or the sepsis - 2 definition described sepsis as the presence of infection in the setting of 2 or more systemic inflammatory response syndrome (SIRS) criteria [Table 1] and severe sepsis and septic shock in the spectrum of its severity. With this old definition, screening with SIRS missed about 1 in 8 patients with infection,[1] yet was positive in non- infection related inflammatory conditions. In 2016 the European Society of Intensive Care Medicine and Society for Critical Care Medicine proposed the third international consensus definitions for sepsis and septic shock (Sepsis-3)[2] and the main premise that brought about this change in definition was due to the current thinking of SIRS as an appropriate response to infection or any other stimulus that activates the inflammatory process rather than a dysregulation of the host response. Nearly half of all patients develop SIRS at least once during their hospitalization rendering SIRS criteria impractical to be used as a screening method.[1] The task force determined that sepsis is not simply infection associated with inflammation, but should be defined as the dysregulated host response to infection leading to life-threatening organ dysfunction. By this definition, the diagnosis would be made when patient’s condition reaches higher acuity, making the term severe sepsis obsolete. For clinical purposes in a data-driven age, to clearly define and screen for organ dysfunction, sequential organ failure assessment (SOFA) [Table 2] and quick SOFA (qSOFA) [Table 3] scoring system is recommended. In the intensive care unit (ICU), the SOFA score has greater predictive validity than SIRS or qSOFA while in general wards qSOFA has similar predictive validity to more complex scores [Table 4]. Clinical criteria for diagnosing sepsis are presence or suspicion of infection along with increase in SOFA points by 2 or more from baseline (for unknown baseline it should be assumed to be 0) and to consider sepsis in patients outside the ICU or when extensive laboratory results are unavailable, 2 or more qSOFA points can be used. Septic shock is a subset of sepsis in which profound circulatory, cellular and metabolic abnormalities are associated with a higher mortality risk. Defining septic shock as hypotension despite fluid resuscitation only implies sepsis with organ dysfunction involving the circulatory system, and this is not the whole truth. Shock is more than that; it is hypoperfusion and severe abnormalities at the organ, tissue, cellular, and metabolic levels. Hence, a low mean arterial pressure (MAP) is not a proxy to hemodynamic instability in septic shock.
Epidemiology
Sepsis is more prevalent in males, advanced age groups and patient with suppressed innate and adaptive immunity. The tendency to get sepsis before the age of 50 could in part be heritable too.[3] There are nearly 850,00 emergency department visits for sepsis annually in United States alone.[4] Sepsis is the third leading cause of mortality and the incidence of this major killer is increasing regardless of which definition one applies. Data from India are very sparse and in the form of infection, microbiological profile, resistance patterns, rather than sepsis which is dysregulated host response. European Prevalence of Infection in Intensive Care – II (EPIC II) study was conducted in 2007 involving 75 countries and based on its data[5] the sepsis-related ICU mortality in India was underestimated due to the nature of the study being a 1-day point prevalence and that not all sepsis-related deaths happen in an ICU setting [Table 5]. EPIC III-point prevalence study was performed on September 13, 2017, the International sepsis awareness day; data from which are awaited. Similarly, Indian intensive care case mix and practice patterns study was a 4-day point prevalence study of 4209 patients from 124 ICUs across India which reported 28.3% patients with severe sepsis or septic shock had ICU mortality of 18.1%.[6] In contrast to a point prevalence study, a 5-year experience from a single center tertiary care the incidence of severe sepsis was 6% in their ICUs out of which 16% were hospital acquired.[7]
Pathophysiology
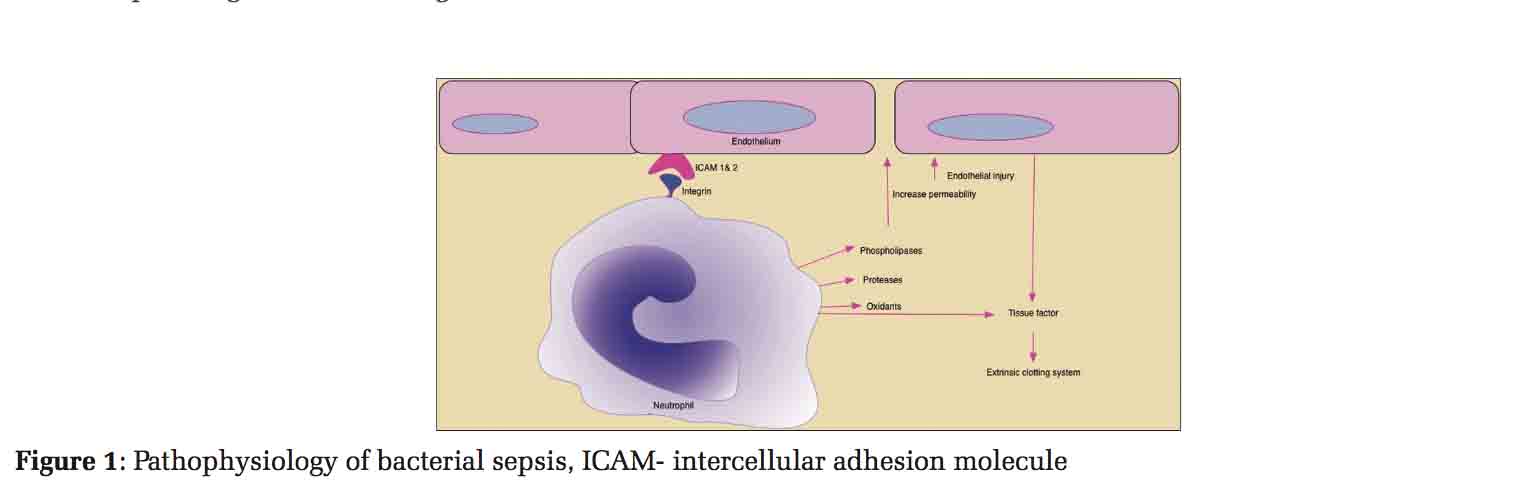
Widespread cellular injury leading to organ dysfunction occurs with florid immune response to infection. Excess proinflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-1 (IL-1) released into the bloodstream leads to progression of local infection to full-blown sepsis. Neutrophils have integrins and selectins that binds to intracellular adhesion molecules (ICAM 1 and 2) on the vascular endothelial cells causing release of oxidants, phospholipases, and proteases. These substances cause increased microvascular permeability and endothelial injury leading to loss of vascular tone. As a reaction from cytokines, the endothelium, neutrophils, and macrophages also release tissue factor (TF) which activates extrinsic clotting system [Figure 1]. Release of other factors like the plasminogen activator inhibitor, platelet activating factor, and Von Willebrand factor amplifies the procoagulant response. Several of the natural anticoagulants such as activated protein C, TF pathway inhibitor, and antithrombin along with fibrinolytic (tissue plasminogen activator) are suppressed leading to overwhelming thrombin formation and microvascular coagulation leading to multi-organ dysfunction. Inflammatory process and coagulation thus are closely linked to sepsis pathogenesis.[8]
Diagnosis and Screening
Sepsis and septic shock can result from an infection anywhere in the body as in pneumonia, urinary tract infection, infected invasive devices, intra-abdominal, and post-surgical infections. It requires active screening in patients who present to the emergency department as well as inpatients to diagnose sepsis. A range of screening tools from a nurse-driven paper checklist to electronic health record-based real-time advisory are available, and the end goal of them all is for early detection possibly even at a pre-hospital stage by the emergency medical services.[9-12] In the age of information and analytics, modified early warning signs[13] and Rothman index[14] can be considered. Some of the tools used are listed in the surviving sepsis campaign (SSC) website (www. survivingsepsis.org). A variety of assessment based on the clinical history is warranted, including temperature, heart rate, respiratory rate, blood pressure, level of consciousness, oxygen saturation, blood cultures, lactate, urea, electrolytes, C-reactive protein, full blood count, kidney and liver function tests, urine analysis and culture, cerebrospinal fluid, wounds, respiratory secretions, or other body fluids that may be the source of infection and finally imaging studies such as chest radiographs and computerized tomography be obtained to confirm a potential source of infection [Table 6].
Lactate
A biomarker that can objectively and accurately measure, reproducibly detect, be universally consistent among all patient populations, and diagnose early does not exist for sepsis yet, but is much needed. At present, lactate measurement and its clearance are used both as a diagnosis and management tool for resuscitation in sepsis. Lactate production in sepsis is multifactorial and incompletely understood. Increased production rather than delayed clearance leads to high lactate levels in endotoxin-mediated sepsis. The source of lactate production is from increased aerobic production that does not take place in the muscle, so other tissues/cells are possibly major contributors.[15] Lactic acid along with hypotension is used to define septic shock because it is a readily available marker of cellular and metabolic abnormality and an independent predictor of mortality in sepsis. Lactate can be used as a screening tool but adds little to the predictive validity when used along with qSOFA. Its greatest utility is as a guide to therapeutic response, an indicator of severity and prognostic tool for mortality as shown in Table 7.[16] There are other sources of lactate such as hepatic, catecholamine, and other drugs that need to be carefully considered.
Management
A Florentine philosopher Niccolò Machiavelli (1469– 1527) half a millennium ago aptly summarized the challenges in sepsis management - “as the physicians say it happens in hectic fever, that in the beginning of the malady it is easy to cure but difficult to detect, but in the course of time, not having been either detected or treated in the beginning, it becomes easy to detect but difficult to cure” and what he stated then, holds true even today.
Major recommendations by SSC for the management of sepsis are grouped in bundles of interventions and are summarized in Table 8. Figure 2 is a comprehensive flow chart for sepsis management. Further detailed treatment issues are discussed below.
Antimicrobial Therapy and Source Control
Before antimicrobial initiation, microbiological cultures from blood, urine, cerebrospinal fluid, wounds, respiratory cultures, or any other sites considered to be the potential source of infection are recommended for pathogen detection as well as for antibiotic resistance testing, unless this causes a substantial delay in starting the antimicrobials. While pan cultures are discouraged, and careful decisions should be made regarding which site to culture, samples that require invasive procedures such as bronchoscopy, thoracentesis, or any open surgery should be procured and need for imaging established at the earliest.
SSC recommends to explore and intervene as early as logistically practical on any anatomic sites that could be the source of infection in patients with sepsis or septic shock, for example, infection related to central venous access or dialysis access, or indwelling Foley catheters should be removed, abscess shouldbedrainedandsurgeryforintra-abdominal source of infection should be planned early. Delay or inadequate source control or inappropriate antibioticsisassociatedwithhighermortality.The time to source control plays a huge role in outcomes, with a direct increase in mortality with each 6 h delay in achieving surgical source control.[17]
The landmark trial by Rivers and group called the early goal-directed therapy (EGDT) was the first study to highlight the recognition of golden hour in sepsis for early antibiotics and resuscitation. This study was a single center prospective, randomized trial that approached sepsis with a protocol based management and showed a staggering absolute risk reduction in mortality by 16%.[18] Since then many studies looking at individual components of this protocol have not been able to demonstrate similar results except for the rapid antimicrobial initiation. In a large retrospective study by Kumar et al., appropriate antibiotic initiation within the first hour of documented hypotension was associated with a survival of 79.9% and every hour delay over the ensuing 6 h, decreased the survival by 7.6%.[19] Similarly, the elapsed time from triage in the emergency department to the administration of antibiotics significantly showed mortality benefit (mortality 19.5% in antibiotic administration < 1 h group vs. 33.2% in >1 h group).[20]
SSC recommends initial empiric anti-infective therapy of one or more drugs that have activity against all likely pathogens (bacterial or fungal or viral) and that reach adequate concentrations into tissues presumed to be the source of sepsis. Combination empirical therapy is recommended for neutropenic patients, patients with multidrug-resistant bacterial pathogens (such as Acinetobacter and Pseudomonas), and patients with severe infections associated with respiratory failure and septic shock. Combination therapy with an extended spectrum beta-lactam and either an aminoglycoside or a fluoroquinolone can be initiated for Pseudomonas aeruginosa bacteremia and a combination of beta-lactam and macrolide for patients with Streptococcus pneumoniae bacteremia. Empiric combination therapy should not be administered for more than 3–5 days and de-escalation to the most appropriate single therapy should be performed as soon as the susceptibility is known. Typically, 7–10 days therapy is indicated, but a longer course may be appropriate in patients who have a slow clinical response, undrainable foci of infection, bacteremia with Streptococcus aureus; some fungal and viral infections or immunologic deficiencies, including neutropenia. Use of low procalcitonin levels can guide discontinuation of empiric antibiotics in patients who initially appeared septic, but have no subsequent evidence of infection. Infectious disease society of America recognizes the importance of the enormous positive impact of SCC on sepsis prevention and treatment. Guidelines on treatment for ventilator-associated pneumonia, hospital-acquired pneumonia, urinary tract infections, skin and soft tissue infection, etc., can be reviewed on http://www.idsociety.org/ PracticeGuidelines/.
SSC recommends early intervention on any anatomic sites that could be the source of infection, as a delay or inadequate source control and even inappropriate antibiotics is associated with higher mortality. In one study with intra-abdominal sepsis, the non-survivors had less than adequate source control (64%) and median time to appropriate antibiotics 23 h versus the survivors who had nearly 91% source control rate and median antibiotic time of 4 h.[21] The time to source control also plays a huge role in outcomes, with a direct increase in mortality with each 6 h delay in achieving surgical source control.[17]
Fluid Resuscitation
In the previously alluded landmark trial by Rivers et al. on EGDT for fluid resuscitation, a protocol based fluid and blood resuscitation with multiple set targets of central venous pressure (CVP), MAP, and mixed venous oxygen saturation (ScVO2) showed significant mortality benefits.[18] Since then, three large randomized trials ProCESS (a randomized trial of Protocol-based Cre for Early Septic Shock), ProMISE (Protocoloized Managemen in Sepsis), and ARISE(The Australasian Resuscitation In Sepsis Evalution) and a patient-level meta-analysis from these PRISM investigators (Protocolized Resuscitation In Sepsis Meta-Analysis) have not shown any mortality benefit from such a protocolized management when compared to usual care.[22-25] Resuscitation to a goal of lactate clearance of at least 10% is non-inferior to ScVO2 target of >70%.[26] While most of the studies on fluid management focus on macro circulatory resuscitation, careful consideration should also be given to microcirculatory (arterioles, capillaries, venules, and lymphatic) flow but future research is indicated in this area.[27]
Choice of Fluids
An ideal fluid should be isotonic to the human plasma, increase the intravascular volume without extravasation into the tissues, maintain the acid- base milieu, should not cause renal toxicity, be cost-effective and hopefully improve mortality. Nothing comes close to this requirement, and currently, the two main choices are crystalloids and colloids. Isotonic crystalloids are the first line agents recommended in the sepsis guidelines, and the most commonly used is 0.9% sodium chloride. This chloride rich solution can lead to non-anion gap hyperchloremic metabolic acidosis and can cause renal vasoconstriction and mesangial contraction leading to decreased glomerular filtration and impaired renal function.[28] Lactated ringers and plasma-lyte though mildly hypotonic resemble the electrolyte concentrations in the plasma and are called as buffered solutions. Stewart’s quantitative approach has revolutionized the thinking behind acid-base physiology. The net strong ion charge on Stewart’s “strong ion difference” SID is the difference between the sum of all strong cation concentrations and the sum of all the strong anions concentrations in plasma. Following normal saline (that contains equal Na and Cl) infusion, the plasma Cl- concentration increases to a greater extent when compared to Na+. This leads to a reduction in the SID and a consequent lowering of the pH. When organic anions such as lactate containing infusions are given, they can be regarded as weak ions that do not contribute to extracellular fluid SID. Despite these differences, the SPLIT trial (The 0.9% saline vs. plasma-Lyte 148 [PL-148] for ICU fluid therapy) which compared the effect of buffered crystalloid solution versus normal saline on acute kidney injury (AKI), found that use of a buffered crystalloid compared with saline did not reduce the risk of AKI (9.6% in the balanced solutions group vs. 9.2% in the 0.9% NaCl group).[29] Colloids although initially was thought to be promising due to their properties of causing increased oncotic pressure and expand intravascular volume without extravasation into the connective tissue space, multiple clinical trials and meta-analyses on hydroxyl-ethyl starch (HES) and normal saline has shown evidence that while there is no difference in mortality, patients who receive HES have higher requirement of renal replacement therapy.[30-33] The saline versus albumin fluid evaluation trial compared albumin and normal saline in critically ill patients, and their subgroup analysis in sepsis and septic shock patients showed some mortality benefit although this did not meet statistical significance.[34] However, albumin Italian outcome sepsis study did not show any difference in organ dysfunction or mortality benefit.[35] Irrespective of the baseline albumin levels in septic patients, albumin is not recommended for sepsis resuscitation as it is not cost effective and there is no mortality benefit based on a recent large meta-analysis.[34,36,37] For resuscitation using blood products, high hemoglobin threshold strategy (>9 g/dl) did not show the difference in mortality when compared to low hemoglobin threshold (<7 g/dl).[38-40]
Maintenance Fluids and De‐resuscitation
Fluid resuscitation in septic shock can be described in four stages - the rescue, optimization, stabilization and evacuation or de-resuscitation phase (ROSE concept).[41] This is a conceptual thinking wherein the first phase is to improve perfusion deficits, the second and the third phase concentrates on maintaining the perfusion while the last phase is for removal of excess fluids used during the first three phases to prevent edema. Fluid resuscitation with large volumes can cause volume overload, worsens already existing increased capillary leak, leads to tissue edema, increases extravascular lung water, increases intra-abdominal pressure leading to compartment syndrome, impedes perfusion to encapsulated organs such as liver and kidneys and causes cerebral edema leading to poor cerebral perfusion pressures. Cautious use of diuretics and renal replacement therapy can help in mobilizing this excess fluid after the acute phase. Positive fluid balance has shown increased mortality in vasopressin and septic shock trial (VASST) and prolonged ventilator need in fluids and catheters treatment trial.[42,43] In a meta-analysis, the patients who were treated with restrictive fluid management, the mortality improved from 33.2% to 24.7%.[44]
Hemodynamic Monitoring
EGDT was based on hemodynamic monitoring of CVP, MAP, and ScvO2. Ideal hemodynamic monitoring should report advanced parameters such as intravascular volume status, fluid responsiveness, global blood flow, myocardial contractility, and cardiac afterload accurately. Some of the available modalities are echocardiography, pulmonary artery catheterization, transpulmonary thermodilution and calibrated and uncalibrated pulse contour analysis in addition to functional testing such as the passive leg raise test and fluid challenge tests. Definite algorithms to guide fluid and pressor therapy using such advance hemodynamic monitoring although exists,[45] there are no studies that have demonstrated improved septic shock related mortality. Instead of targeting distinctvaluesofCVPandScVO2theSSCguidelines now recommend to re-assess volume status and tissue perfusion (6-h bundle) by repeated focused exam and lactate clearance.
Vasoactive Agents in the Management of Septic Shock
The clinical criteria for identifying septic shock patients are vasopressor requirement to maintain MAP of 65 mmHg or greater and serum lactate level >2 mmol/l (>18 mg/dl) in the absence of hypovolemia.[16] Vasopressors such as norepinephrine, dopamine, phenylephrine, epinephrine, and vasopressin/terlipressin as well as inotropes such as dobutamine and milrinone are common choice of vasoactive agents. Some of them have overlapping functions. The first line drug recommended in septic shock is norepinephrine, based on multiple randomized controlled studies and meta-analysis comparing dopamine and norepinephrine. Use of norepinephrine was found to be superior in the reduction of mortality and adverse cardiac events.[46] Use of dobutamine can increase cardiac index, however, lowers MAP and increases oxygen extraction. Experts suggest there is a role for dobutamine in pump failure due to septic cardiomyopathy, as manifested by elevated cardiac filling pressures and low cardiac output. Epinephrine has potent inotropic and vasoconstrictive effects but less commonly used as a first line agent in septic shock, which is typically associated with hyperdynamic circulation. When epinephrine was compared to norepinephrine plus dobutamine, there were no significant mortality benefits[46] although it was associated with greater cardiac index.[47] Vasopressin is a useful drug in refractory shock, however, the VASST study could not show any beneficial effects.[42]
Organ Support
For patients with sepsis-induced adult respiratory distress syndrome, SSC suggest using higher positive end-expiratory pressure (PEEP) over lower PEEP, lower over higher tidal volume setting on the mechanical ventilator, and prone positioning over supine. SSC also strongly recommends a protocolized approach to blood glucose management in ICU patients with sepsis, commencing insulin dosing when two consecutive blood glucose levels are >180 mg/dL and maintaining upper blood glucose level ≤180 mg/dL rather than ≤110 mg/dL. SSC suggest against using intravenous hydrocortisone to treat septic shock patients if adequate fluid resuscitation and vasopressor therapy are able to restore hemodynamic stability. If this is not achievable, intravenous hydrocortisone at a dose of 200 mg per day can be considered in refractory shock. Further considerations for the use of steroids can be reviewed in guidelines for critical illness-related corticosteroid insufficiency.[48] In patients with acute kidney injury related to septic shock, optimal timing of starting renal replacement therapy is still up for debate.[49,50] Only about 59% of patients with renal failure due to sepsis recover renal function.[51] SSC recommends that the goals of care are incorporated into treatment and end-of-life care planning, utilizing palliative care principles where appropriate.
Nutrition
In 2016, Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition (ASPEN) developed guidelines for feeding in the critically ill patients. Assessment of nutritional status using scoring systems such as NUTRIC score and NRS 2002 should be made, and comorbid conditions should also be taken into account. Energy requirement must be calculated using indirect calorimetry or predicted equations. On average a critically ill patient would need 25–30 kcal/kg and adequate proteins of 1.2–2.0 g/kg/day. Patients with high nutrition risk should be provided with >80% estimated requirements along with a high dose of protein and should be monitored for refeeding syndrome. High protein, hypocaloric feeds to preserve lean body mass should be used in obese patients to minimize complications of over- feeding. Early oral or enteral feeding as tolerated is recommended rather than fasting or glucose based intravenous therapies. Trophic feeds started during the initial acute phase of sepsis (≤500 kcal/day) should be advanced after 24–48 h. Bowel sounds and evidence of bowel function is not required for the initiation of enteral nutrition. Post-pyloric feeding is preferred in patients with high aspiration risk and failed gastric feeds in the past. Holding of enteral nutrition for gastric residual volumes of <500 ml should be discouraged and volume based feeding to maximize provision of goal calories is supported. In the presence of persistent diarrhea, use of mixed-fiber, and peptide-based formula can be considered. Use of parenteral nutrition in the first 7 days is also not advocated regardless of nutritional risk. There are, currently, no immunomodulating supplementation or probiotics recommended by the SSC guidelines, but they do recommend providing antioxidants and trace minerals in safe doses.[52]
Future Directions
The paradigm shift and focus for future advances and improvements in the care of patients with sepsis are mainly in three areas of 1. Earlier recognition- in the prevalence study by Novosad et al., nearly 72% of sepsis patients had either a health-care factor (i.e.,≥2 days in a nursing home, long-term or another acute care hospital, receipt of intravenous antimicrobials, peritoneal or hemodialysis, surgery, total parenteral nutrition, chemotherapy, wound therapy, or presence of a central venous catheter) or chronic disease condition in the prior 1 month of their sepsis presentation.[53] This provides a wide opportunity for sepsis prevention and early intervention. 2. More specific and more sensitive screening tools and biomarkers-use of machine learning algorithms and computer decision support systems along with the growing use of electronic health records bring better prospects in screening and decision-making for individual patients. For a biomarker to be successful in identifying sepsis, it should objectively measure and evaluate accurately and reproducibly, has to be universal and apply to the entire population, be able to distinguish from other processes with great certainty and be able to identify early in the disease onset. Due to the complex pathophysiology of sepsis, any particular biomarker falls short in accurately diagnosing it. Angiopoietin-1, Angiopoietin-2, CD-64, triggering receptor expressed on myeloid cells-1, IL-6, IL-8 TNF-α, regulatory T cells, Programmed cell death receptor-1/Programmed cell death receptor-L1, B and T lymphocyte attenuator, Cytotoxic T-Lymphocyte antigen-4, gene expression profiles, etc., although are promising they just reflect only a part of a phase of the disease process, so looking at a combination of readouts reflecting the various aspects of the host response may be more promising.[54] 3. Optimizing recovery-patients with sepsis syndromes have significantly worse outcomes with lower health- related quality of life. The rate of a major illness recurring and/or mortality is also increased in the year after ICU discharge, with mortality ranging from 26% to 63% at 1-year post discharge in long-stay ICU patients (≥48 h).[55] There is a major yet unexplored role for post-sepsis discharge care navigation that would address issues such as physical rehabilitation, delirium, and cognitive dysfunction.
Preventive Measures
Benjamin Franklin’s quote, “an prevention is worth a pound of cure” bears tremendous relevance on sepsis preventative measures. Vaccination, hand washing, wound care, appropriate storage of antibiotics, removal of catheters when not in use, universal precautions, appropriate isolation, multidisciplinary rounding, early symptom recognition, and education in high- risk patients such as diabetics, neutropenic, and postpartum patients are some great measures. Any initiative designed to improve adherence to the sepsis guidelines and thus improve performance in sepsis core measures requires an institution- specific, strategic, and planned approach. A transdisciplinary team charged with the functions of raising sepsis awareness, developing sepsis focused educational programs, establishing a care pathway model and monitoring compliance and adherence to the sepsis bundles can help improve the sepsis outcomes. In a global, prospective, observational, quality improvement study of adherence to the SSC bundles in patients with severe sepsis or septic shock, compliance to 3-h bundle resulted in a 40% reduction in mortality and 6-h bundle of 36% reduction in mortality.[56] Some major areas of considerations for quality improvement initiatives in individual institutions are the patient populations cared for in the facility, available resources and the culture of practice in the facility. With the diligent engagement of all stakeholders, a positive effect on sepsis-associated mortality can be achieved.




Subscribe now for latest articles and news.