Introduction
People with hematologic malignancies (PWHM) are at increased risk of severe complications and death from the coronavirus disease of 2019 (COVID-19) due to a combination of innate immunosuppression inherent to the disease process, immunosuppressive treatments, and existing comorbidities. The disease process within the spectrum of malignant hematologic conditions 1 and their treatments is also highly heterogeneous, as both impaired immune response and excessive activation of cytokines and T-cells have been implicated in severe COVID-19 2. Specific hematologic malignancies (HM), including B-cell non-Hodgkin’s lymphomas (NHL) 3 and chronic lymphocytic leukemia (CLL), 4 are associated with very low response rates to COVID-19 vaccination 5 and often require booster doses6 to demonstrate seroconversion to vaccination. Likewise, PWHM who are receiving medications that target B-cells, including rituximab and other B-cell depleting monoclonal antibody therapy 7, 8 as well as Bruton’s tyrosine kinase inhibitors (BTKi) 9 have an extremely low rate of seroconversion after vaccination. In contrast, people with myeloproliferative malignancies 10, solid tumors (ST) on antineoplastic therapy 11, and human immunodeficiency virus infection (HIV) on antiretroviral therapy (ART) 12 have higher rates of detectable antibodies after vaccination against COVID-19, suggesting that humoral immunity may be better preserved in these individuals. Given the ongoing shifts in predominant COVID-19 strains and uncertainties regarding the consequences of infection and reinfection 13, it is crucial to identify individuals who are at a high risk of non-response to COVID-19 vaccination.
Methods
We conducted a retrospective study of adult patients seen at the hematology and medical oncology clinic at Virginia Mason Medical Center between May 2020 and July 2022. All patients who had received two doses of mRNA-1273 (Moderna) or the BN162b2 (Pfizer) vaccine were included in this study. Patients without a complete vaccination history were excluded from analysis. Counseling was provided and verbal consent obtained for the collection of blood to assess COVID-19 antibody response to vaccinations as part of routine medical care. Laboratory data and vaccination history were reviewed in the electronic medical record and deidentified prior to analysis. This study was exempt from Institutional Review Board review and monitoring as research was performed on deidentified information collected from the electronic medical record.
Anti-COVID-19 antibodies were assessed with the semi-quantitative Roche Diagnostics SARS-CoV-2 Spike Ab total antibody test against the spike glycoprotein. A serum spike antibody level ≥ 0.8U/mL was considered evidence of seroconversion as defined by the manufacturer 14 and the upper limit of detection for this assay was 250 U/mL 15. Antibody levels were assessed 28 days after the patients’ initial 2-dose vaccination series and reassessed between 28 and 35 days after booster vaccination. Breakthrough infection was defined as a positive polymerase chain reaction (PCR) COVID-19 test in a patient who had received at least 2 vaccine doses. Data regarding COVID-19 breakthrough infections were reviewed in the patients’ medical records until the data collection cutoff date of November 11, 2022. We used a Mann-Whitney test to calculate associations of continuous variables and the chi-square test to examine association between categorical variables. All p values < 0.05 from statistical tests were considered significant.
Results
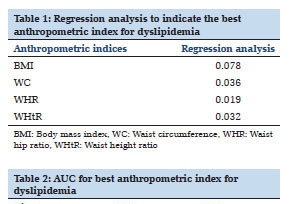
A total of 299 vaccinated individuals were included in the study, of which 202 had HM, 48 had HIV without diagnosed cancer, 17 had both HIV and cancer, 12 had ST, 10 had autoimmune diseases, and 10 had other conditions (Table 1 ). The median age was 68 years (range, 25-98 years) and 201 individuals (66%) were male. In people with HIV (PWH), the median CD4+ count was 541 cells/mm3 (range 4-2260 cells/mm3) and the median HIV viral load < 40 copies/mL (range < 40-700,000 copies/mL). Most PWH were adherent to ART as evidenced by an undetectable viral load (56 of 65 participants). CD4+ counts were > 200 cells/uL for 64 of 65 individuals. Among the 17 people with a dual diagnosis of HIV and cancer, 11 had Kaposi Sarcoma (KS), an AIDS-defining cancer. Most patients (220 of 299, 73.6%) were receiving systemic therapies to treat their diseases.
|
|
All patients |
Seroconversion |
No seroconversion |
P value |
|
Variable |
|
|
|
|
|
Number |
299 |
248 (83%) |
51 (17%) |
|
|
Age, median (IQR) in years |
68 (59-75) |
67 (58-75) |
70 (63-74) |
0.155 |
|
Sex |
|
|
|
1 |
|
Men |
195 (65%) |
162 (83%) |
33 (27%) |
|
|
Women |
104 (35%) |
86 (83%) |
18 (27%) |
|
|
WBC: Median (IQR), ×109/L |
6.2 (4.6-8.2) |
6.1 (4.6-7.9) |
7.1 (4.8-10.2) |
0.064 |
|
ANC: Median (IQR), ×109/L |
3.3 (2.3-4.6) |
3.3 (2.3-4.5) |
3.3 (2.1-5.1) |
0.748 |
|
ALC: Median (IQR), ×109/L |
1.5 (1.0-2.4) |
1.5 (1.0-2.3) |
1.5 (8.6-3.4) |
0.992 |
|
|
|
|
|
|
|
Disease Category |
|
|
|
<0.001 |
|
Autoimmune |
10 |
5 (50%) |
5 (50%) |
|
|
CLL |
44 |
29 (66%) |
15 (34%) |
|
|
Lymphoid malignancies |
72 |
48 (67%) |
24 (33%) |
|
|
Plasma cell dyscrasias |
43 |
37 (86%) |
6 (14%) |
|
|
HIV and cancer |
17 |
16 (94%) |
1 (6%) |
|
|
HIV alone |
48 |
48 (100%) |
0 (0%) |
|
|
Myeloid malignancies |
43 |
43 (100%) |
0 (0%) |
|
|
Solid tumors |
12 |
12 (100%) |
0 (0%) |
|
|
Other |
10 |
10 (100%) |
0 (0%) |
|
|
|
|
|
|
|
|
Treatment Category |
|
|
|
<0.001 |
|
ART alone |
46 |
46 (100%) |
0 (0%) |
|
|
ART with other therapy |
15 |
14 (93%) |
1 (7%) |
|
|
Bendamustine and rituximab (BR) |
18 |
11 (61%) |
7 (39%) |
|
|
BTK inhibitors |
22 |
14 (64%) |
8 (36%) |
|
|
Chemotherapy |
21 |
18 (86%) |
3 (14%) |
|
|
Hydroxyurea |
14 |
14 (100%) |
0 (0%) |
|
|
Immunosuppression |
4 |
3 (75%) |
1 (25%) |
|
|
Multiple myeloma treatments |
32 |
26 (81%) |
6 (19%) |
|
|
PD-L1 agents |
6 |
5 (83%) |
1 (17%) |
|
|
R-CHOP |
19 |
10 (53%) |
9 (47%) |
|
|
Rituximab |
10 |
3 (30%) |
7 (70%) |
|
|
Other |
12 |
12 (100%) |
0 (0%) |
|
|
No treatment |
80 |
72 (90%) |
8 (10%) |
|
Abbreviations: AIDS, acquired immunodeficiency syndrome; AML, acute myeloid leukemia; ALC, absolute lymphocyte count; ART, antiretroviral therapy; AU, arbitrary units; BTKi, Bruton’s tyrosine kinase inhibitors; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; COVID, coronavirus disease; HIV, human immunodeficiency virus; IQR, interquartile range; MDS, myelodysplastic syndrome; MGUS, monoclonal gammopathy of undetermined significance; MPD, myeloproliferative disorders; PD-L1, programmed death-ligand 1; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
After their initial vaccination series, 247 out of 299 individuals (81%) had detectible COVID-19 antibodies. Disease subtypes and treatment categories were grouped according to Table 1. Seroconversion rates decreased with older age but were not significantly associated with white blood count (WBC), absolute neutrophil count (ANC), or absolute lymphocyte count (ALC) (Figure 1 A).

Seroconversion rates were lowest in people with autoimmune diseases (5 of 10, 50.0%), CLL (29 of 44, 65.9%), and lymphoid malignancies (48 of 72, 66.7%) (Figure 1 B). All PWH alone, myeloid malignancies, ST (n = 103) and 86% of patients with plasma cell dyscrasias (PCD) (37 of 43) demonstrated seroconversion after primary 2-dose vaccination (Figure 1 B). Patients receiving anti-B-cell monoclonal antibody therapy and Bruton’s tyrosine kinase inhibitors (BTKi) had significantly lower rates of seroconversion (Figure 2).

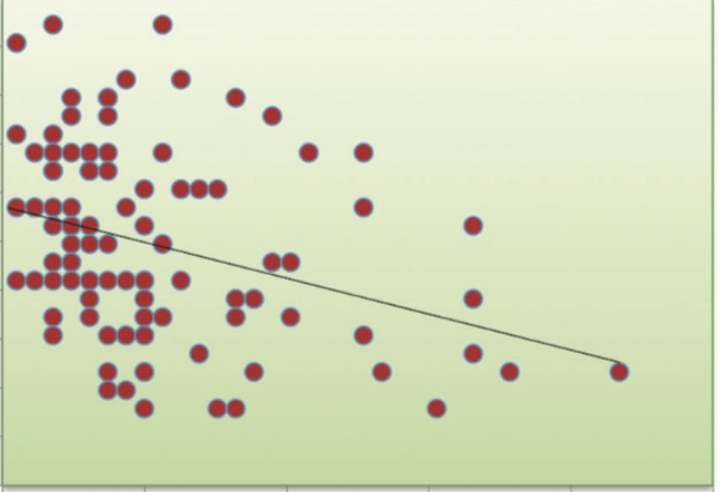
PWH alone and people with ST had higher anti-spike antibody levels (Figure 3 A), whereas those receiving anti-B-cell therapy had lower antibody levels (Figure 1 C). PWH and those with a dual diagnosis of cancer had intermediate antibody levels (Figure 3 B). In the 51 individuals who did not seroconvert after their primary vaccination course, roughly 50% (25) achieved seroconversion after receiving one booster dose. One individual who did not seroconvert with 5 vaccine doses successfully demonstrated seroconversion after obtaining a 6th vaccine dose. Those with prior exposure to anti-B-cell monoclonal antibody therapy had lower anti-spike protein antibody titers (Figure 1 C). However, there was no significant difference (p = 0.577) in rate of breakthrough infections in each quartile of neutralizing antibody titer (Figure 4). Of the 299 individuals we reviewed, 46 (15%) had a documented COVID-19 infection.


Discussion
In this retrospective study, we analyzed serological response to COVID-19 vaccination in individuals with HIV, HM, ST, and other conditions to better understand the immunogenicity of vaccination in a diverse patient population. Most of our data was collected while COVID-19 was actively undergoing antigenic drift which resulted in the evolution of novel COVID-19 variants and shifts in the distribution of variants in our geographic area and in other areas of the United States (US) 16. During our study period, Omicron and Delta variants were the predominant circulating strains. In addition, tixagevimab/cilgavimab was given as pre-exposure prophylaxis for patients with qualifying medical conditions or treatments that may result in immunosuppression, but we were unable to study the effectiveness of tixagevimab/cilgavimab due to the low incidence of symptomatic COVID-19 infection in our study population. In concordance with prior studies 1, 3, 4, 5 that have observed low rates of seroconversion in patients with HM and subsequent seroconversion after booster vaccination 6, we also observed that booster vaccinations can result in detectable antibodies in approximately half of the patients who were seronegative after their initial vaccination course. However, we did not find a significant difference in rates of breakthrough COVID-19 infection between disease categories or treatment categories.
There was no significant association between antibody titer and rates of breakthrough COVID-19 infection within the range of the assay (up to 250 U/mL) that we used to measure anti-spike protein antibody levels. Subsequent studies have suggested using an anti-spike protein antibody titer of 5000 U/mL as a threshold for detecting an adequate antibody response 17 Although US Food and Drug Association (FDA) Emergency Use Authorization (EUA) currently recommends against using the semiquantitative COVID-19 antibody test to assess immunogenicity for clinical purposes 18, emerging data suggest that antibody level testing can help identify people who may be at a high risk of infection and who would likely benefit from additional booster vaccinations. A cross-sectional study in the United Kingdom 17 analyzing more than 3,000 patients with cancer showed that they were more likely to have undetectable antibody levels compared to the general population, and that patients with leukemia or lymphoma had the lowest antibody titers among the cancer patients studied. These patients with cancer who had an undetectable antibody response were found to be at a much higher risk for breakthrough infection and hospitalization, supporting the use of antibody testing to identify patients at high risk for vaccine non-response and serious complications related to COVID-19 infection.
Our study revealed that those who did not seroconvert after they completed a primary vaccination series and booster doses as recommended by the US FDA for immunocompromised individuals 19 may develop antibodies with additional vaccine doses. Our data are supported by a larger nationwide cohort study conducted in Singapore which showed that cancer patients received significant protection against severe COVID-19 with a third dose of vaccine and were further protected after a fourth dose of vaccine 20. Although the benefit of fifth and sixth doses of vaccine is still uncertain, one of our patients acquired additional vaccine doses outside of existing guidelines and successfully seroconverted after six total doses of vaccine.
Currently, adoption of COVID-19 vaccines in the US among those who qualify for COVID-19 vaccinations, especially booster vaccination, remains low (17% as of May 2023) 21 and uptake among various groups that make up the general population is extremely heterogenous. In the US, surveys performed by the Centers for Disease Control and Prevention (CDC) show lower bivalent booster adoption rates in younger adults, people living in rural areas, Americans without health insurance, and in Black and Hispanic Americans. Studies analyzing cancer patients showed that counterintuitively, people with more aggressive cancers were less likely to be vaccinated and that patients with comorbid diseases in addition to cancer were also less likely to be vaccinated 22, even though their comorbid cardiovascular, gastrointestinal, or autoimmune diseases likely placed them at risk for severe COVID-19 disease. This highlights the importance of utilizing antibody testing in conjunction with consideration for social determinants of health, including level of education and other socioeconomic factors, to identify individuals at highest risk of vaccine hesitancy, risk of vaccine non-response, and complications from COVID-19 disease.
Our study has several limitations that are important to consider. Our data set contained a significant number of antibody titers that were above the primary measuring range (0.40-250 U/mL) of the assay we used to measure antibody levels. As a result, we were unable to study the significance of high antibody levels above the limit of 250 U/mL due to limitations of the semiquantitative assay that we used 14 Subsequent protocols have been developed to automatically dilute samples above 250 U/mL to allow an upper limit of quantification as high as 25000 U/mL 15. Nevertheless, 158 patients (53%) in our study population had antibody titers in the measurable range (0.40-250 U/mL) of this test. Multiple patients qualified for pre-exposure prophylaxis with tixagevimab/cilgavimab administered under the US FDA EUA 17, which might have influenced the rate of breakthrough COVID-19 infection. However, shortly after our data collection concluded, the EUA for tixagevimab/cilgavimab was revised 23 to revoke authorization for use while the frequency of non-susceptible variants is greater than 90%. We did not collect data on the use of tixagevimab/cilgavimab as its efficacy is still uncertain 24.
In general, our patients promptly reported positive COVID-19 test results or significant exposure to COVID-19 to their oncologist, but cases of asymptomatic COVID-19, unconfirmed cases, and unreported cases may have been missed. Additionally, we did not study the cellular response to vaccination, which may play an important role in immunity against COVID-19 infection and reinfection 25. Additional limitations of our study include a limited sample size and potential selection bias in our single institution study, where patients who were concerned about COVID-19 were more likely to obtain vaccination, consent to blood draws, and undergo anti-COVID-19 antibody testing. It should also be noted that our practice consists of a variety of diseases, which limits the statistical power of our study due to the presence of several disease and treatment groups with a limited number of patients.
Despite these limitations, our study underscores the variability in serological response to COVID-19 vaccination among individuals with HM, ST, and HIV. Notably, the incidence of breakthrough infections did not correlate significantly with the degree of seroconversion, highlighting the complex interplay between immune response and clinical outcomes in these populations. Nevertheless, our study illustrates the potential utility of serological testing to identify extremely high-risk individuals and guide personalized vaccination strategies. Moving forward, further studies are needed to address the limitations of our study, including its retrospective nature, limited sample size, potential selection bias, and the absence of cellular immune response data. Larger, multicenter, prospective studies are necessary to validate these findings and enhance our understanding of the immune response to COVID-19 vaccination in these complex patient populations.
Conclusion
Our study supports the potential utility of COVID-19 serological testing to identify individuals who may have diminished response to COVID-19 vaccination and to help physicians develop personalized COVID-19 vaccination recommendations. Although the rate of breakthrough infections did not correlate significantly with seroconversion rates in our study, serological testing can also help identify individuals who may still be at high risk of developing severe COVID-19 disease. With ongoing uncertainties regarding the consequences of infection and reinfection, continued research is needed to refine our understanding of immune response in high-risk patient populations and improve their protection against COVID-19.
Acknowledgments
The authors thank Ms. Kristin Nicholson, RN, for her indispensable and indefatigable assistance in the clinical care and follow-up of our clinic patients. We also acknowledge and thank Virginia M. Green, PhD, for editing and preparing this manuscript.