

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2020.v06i03.002
Year: 2020, Volume: 6, Issue: 3, Pages: 7-13
Original Article
Subrata Das1, Anirban Roy2
1Associate Professor, Department of Obstetrics and Gynaecology, ESI PGI MSR, ESIC Medical College and ESIC Hospital and ODC (EZ), Diamond Harbour Road, Joka, Kolkata, West Bengal,
2Associate Professor, Department of Psychiatry, Institute of Psychiatry Centre of Excellence, Institute of Post Graduate Education and Research, Kolkata, West Bengal, India
Address for correspondence:
Dr. Subrata Das, J/6, Banerjee Para, Kamdahari, Garia, Kolkata - 700 084, West Bengal, India. Phone: +91-9432326356. E-mail: [email protected]
Background: Intrahepatic cholestasis (IHC) is usually a disease of late pregnancy and presented with intense pruritus with elevated liver enzymes and multifactorial in origin. Common association of IHC with meconium-stained amniotic fluid (MSAF) and adverse perinatal outcome is our area of concern.
Objectives: The aim of the study is to know the bile acid as a predictor for adverse perinatal outcome in pregnancy complicated by IHC.
Materials and Methods: The present observational study was conducted on 76 women, who were selected consecutively from the outpatient department of antenatal care from a tertiary care hospital of East India from January 2019 to December 2019. Women of more than 28-week gestation with pruritus of whole body and deranged liver function tests were included in the study. Descriptive analysis was used to derive mean and standard deviation from demographic data. The serum bile acid level was compared with frequencies of MSAF and adverse perinatal outcomes, level of significance was measured by calculating P-value.
Results: Incidence of IHC was 3.3%, with “mean of age” and “gestational weeks” of women of 25.25 ± 3.61 years and 36.11 ± 2.75 weeks, respectively. Elevated bile acid level in relation to the presence of MSAF and each adverse perinatal outcome were highly significant (< 0.01) found. The relationship was not significant (>0.05) in comparison of gestational age with MSAF.
Conclusions: Maternal serum bile acid level can be utilized as a predictor for MSAF. However, more research is needed to predict poor fetal outcome in the pregnancy which is complicated by IHC.
KEY WORDS: Intrahepatic cholestasis in pregnancy, meconium-stained amniotic fluid, perinatal outcome, serum bile acid level.
Intrahepatic cholestasis (IHC) is the most common hepatic disorder during pregnancy.[1] It is reversible disorder, characterized by intense pruritus that involves whole body including palm and sole, with consequent sleep deprivation.[2] IHC usually is found at the second or third trimester of pregnancy along with abnormally high serum aminotransferases and/or raised bile acid level.[3,4] The marginal rise of total bilirubin (TB) is often associated with IHC but symptoms typically resolve in 48 h postpartum but recurs on subsequent pregnancies.[5] Ethology of exact pathology is largely unknown.[6] Diagnosis of IHC is done by exclusions, in the absence of demonstrable hepatic or gallbladder disease. For the purpose of diagnosis, serum level of liver enzymes, bile acid, bilirubin with viral marker for hepatitis B and C assay, and upper abdominal sonography are needed. Symptoms of mild upper abdominal pain, dark colored urine, and steatorrhea were often associated and malabsorption of fat-soluble vitamins, often leading to depletion of Vitamin K-dependent clotting factors which further enhance the risks of postpartum hemorrhage (PPH).[2]
Incidence of IHC varies worldwide, it is largely influenced by geographical and environmental distribution, ethnicity, genetic, and hormonal changes.[7] Women with multiple pregnancy are more prone to develop IHC then singleton pregnancy.[8] Highest incidence of IHC is reported from Chile (1–14%) and Poland and Spain (12%), followed by Sweden (1–1.5%), England (0.7%), and France (0.2%).[3,4,5,7] In India, it’s prevalence of 1–5% is reported from different studies, the variations are mostly due to relevant geographical variation of incidence.[1,6]
Maternal treatment with ursodeoxycholic acid (UDCA) and Vitamin K is the only available treatment option.[9] Treatment with UDCA may cause significant relief of pruritus and improved biochemical parameters. Due to possibilities of adverse pregnancy outcome, several authors advocated for termination of pregnancy at 38 weeks or before,[1] depending on raised bile acid levels.
In IHC, perinatal outcome can be complicated by meconium-stained amniotic fluid (MSAF), preterm birth, fetal asphyxia with low Apgar score, and increased incidences of neonatal intensive care (NICU) admission or fetal demise.[10] There are more incidences of preterm labor, preterm premature rupture of the membranes (PPROM) and cesarean section (LUCS) are seen in IHC. Some study shows that there is significant association between markedly increased bile acid level (>40 μmol/L) and presence of MSAF.[8,10,11] The aim of our study was to know if IHC in pregnancy increases the risk of adverse perinatal outcome.
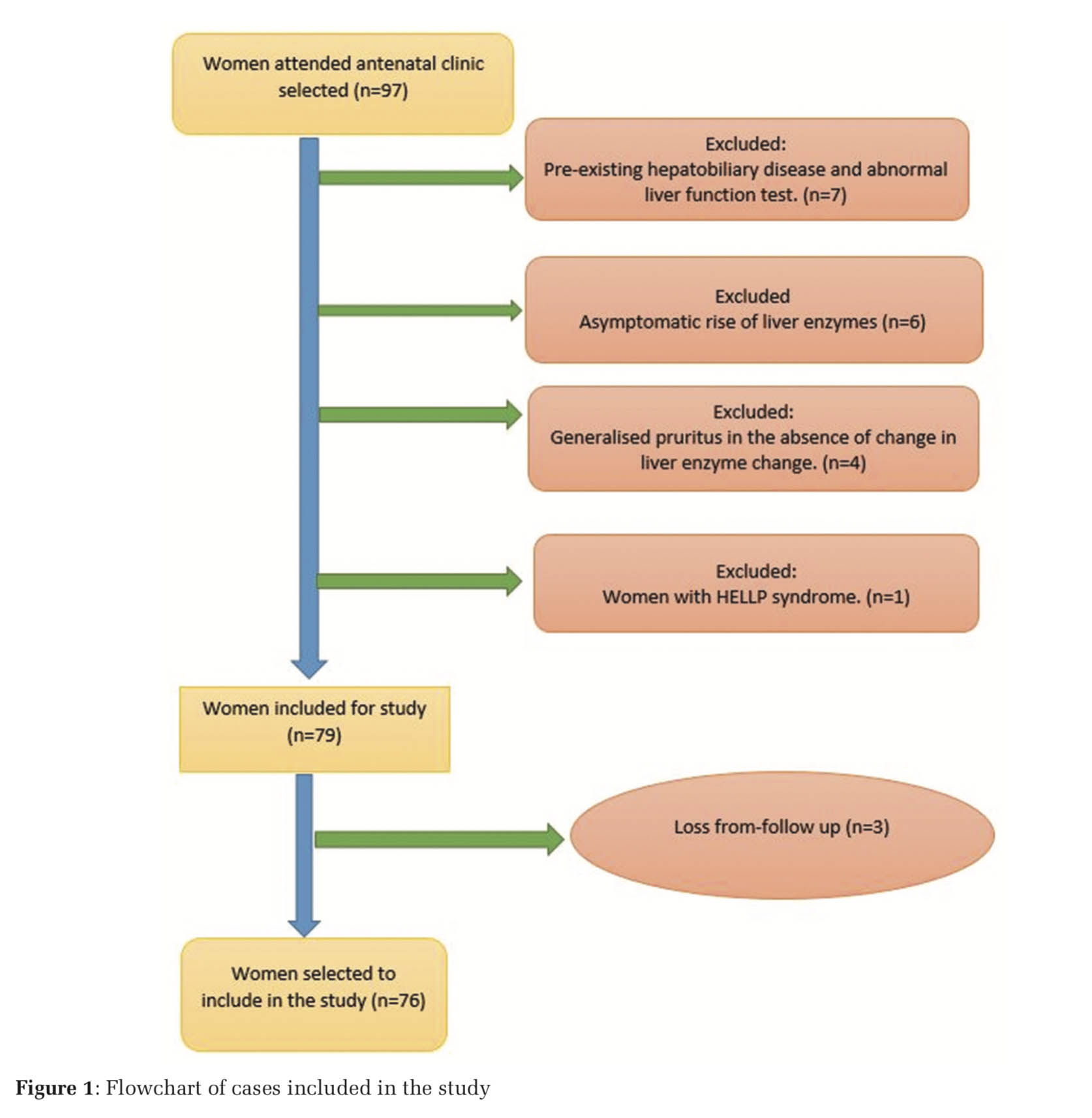
It was an observational study conducted in a tertiary care hospital of East India during the 1-year period of January 1, 2019–December 31, 2019. Seventy-six antenatal women of more than 18 years’ age and more than 28 weeks of gestation with diagnosis of IHC were subject in this study. Method for collecting data used, was complete enumeration method within the study period. IHC was diagnosed in the women with pruritus involving the whole body including palm and sole which were our study sample. The pruritus of the women was worsening at night times and associated with deranged liver function test (LFT). Women with dermatological disorders, hepatobiliary, gallbladder disease or viral hepatitis were excluded from the study. The pregnant women of IHC with singleton pregnancy, irrespective of parity, non- alcoholic and normotensives, and absence of AFP (acute fatty liver of pregnancy) or HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count in combination) were included in the study. Studied women were treated with 600–900 mg/day of UDCA depending on the severity of symptoms (Figure 1).
All women were put under dermatological consultation to exclude the presence of dermatological disease and also upper abdominal sonography was used to rule out hepatobiliary and gallbladder pathologies. Hepatitis B surface antigen and/or antibody for hepatitis C screen positive women were also excluded from the study. The pregnancy-specific ranges as described by Bhattacharrya and Kavita in their study[12] were utilized to see the abnormalities in the LFT.
Detailed history and findings of clinical examination were recorded and women were monitored by daily fetal movement count, cardiotocography, biophysical profile, and fetal ultrasonography at usual interval for fetal surveillances. As per international guideline, women were subjected to standard treatment protocol. Data were recorded regarding maternal events of PPROM and mode of delivery (i.e., cesarean section, instrumental, or vaginal delivery) and perinatal outcome as fetal distress, Apgar score at 5th min, presence of MSAF, and NICU admission. RCOG guidelines were followed for diagnosing non-reassuring fetal heart rate pattern to document fetal distress. In our study, we considered fetal distress, low Apgar score (i.e., < 7 in 5 min), preterm birth, admission in NICU, and stillbirth as adverse perinatal outcomes. These adverse perinatal outcomes were treated as unexpected outcomes in the study.
Descriptive statistics were used to analyze data for mean, standard deviation, percentage, and proportion and are presented in tabular and graphical formats. Women were labeled as IHC in studied group when one or more parameter had crossed upper margin of pregnancy specific level of LFT (12), that is, serum aspartate aminotransferase (AST) (>59 U/L), alanine aminotransferase (ALT) (>72 U/L), TB (>1.3 mg/dl), total bile acids (TBA) (>10 μmol/L), and serum alkaline phosphatase (>126 IU/L). Women were categorized in four groups on the basis of serum total bile acid level (TBA), that is, normal level (< 10 μmol/L), mildly increased (10- 39.9 μmol/L), moderately increased (40-100 μmol/L), and severely increased (>100 μmol/L). The normal level (< 10 μmol/L) was used as reference level for comparison. The presence of MSAF was compared with the women of elevated total bile acid (TBA) and the groups of women according to gestational age. From the Pearson Chi-square test, P-value was calculated. P < 0.05 and < 0.01 were considered as statistically significant and highly significant, respectively. Collected data were analyzed in the statistical software of SPSS 24th version.
A total of 2303 women were delivered within our study frame in which 76 women had a diagnosis of IHC in pregnancy. Hence, incidence was 3.3% in the study group.
According to age, women were divided into four categories. The maximum number 39 (51.3%) women were from 21 to 25 years’ age group and 25 (32.9%) were from 26 to 30 years’ age group. This was followed by 7 (9.2%) and 5 (6.6%) from more than 30 years and less than 20 years’ age group. The mean age of women was 25.25 ± 3.61 years in the study subjects (Table 1). Mean gestational age was 36.11 ± 2.75 years among the studied women (Table 1).
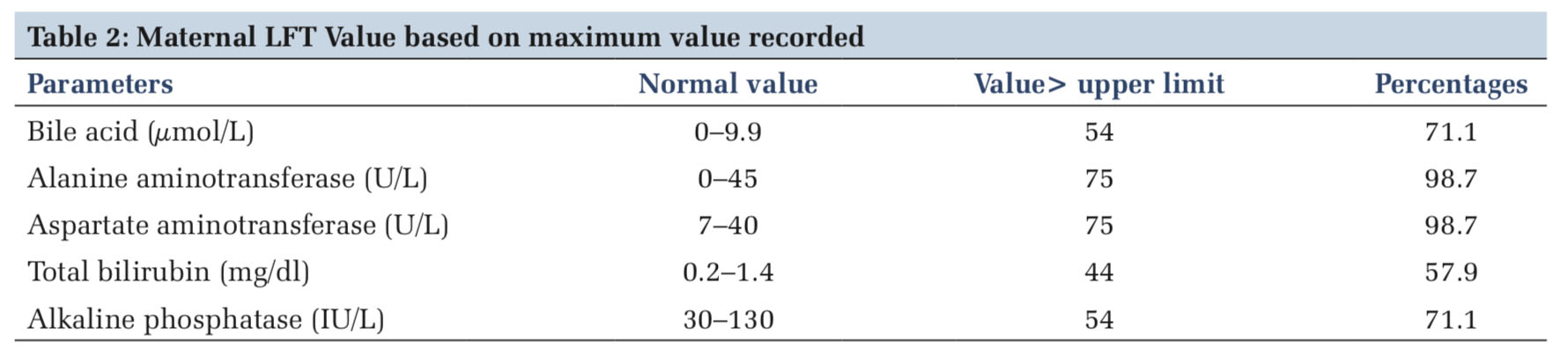
With one exception, all the other women, that is, 75 (98.7%) had increased serum level of ALT and AST. The serum level of TB and alkaline phosphatase was increased to 44 (57.9%) and 54 (71%), respectively. Bile acid level of 54 (71.05%) women was higher than normal limit (Table 2).
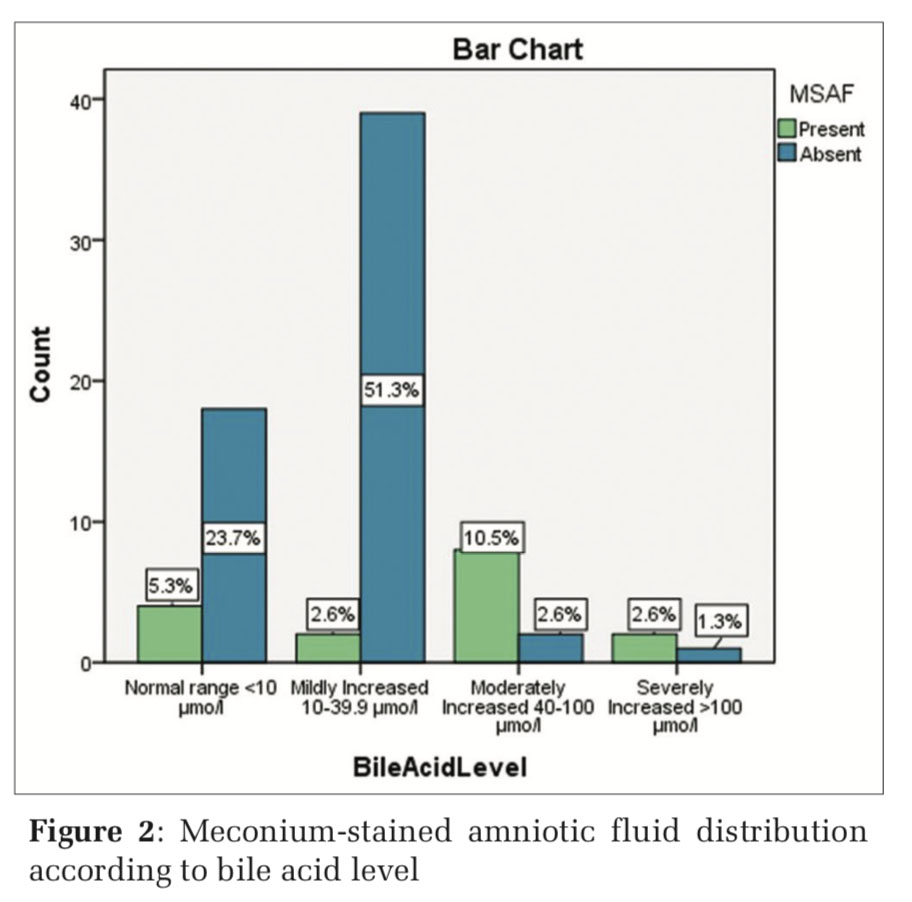
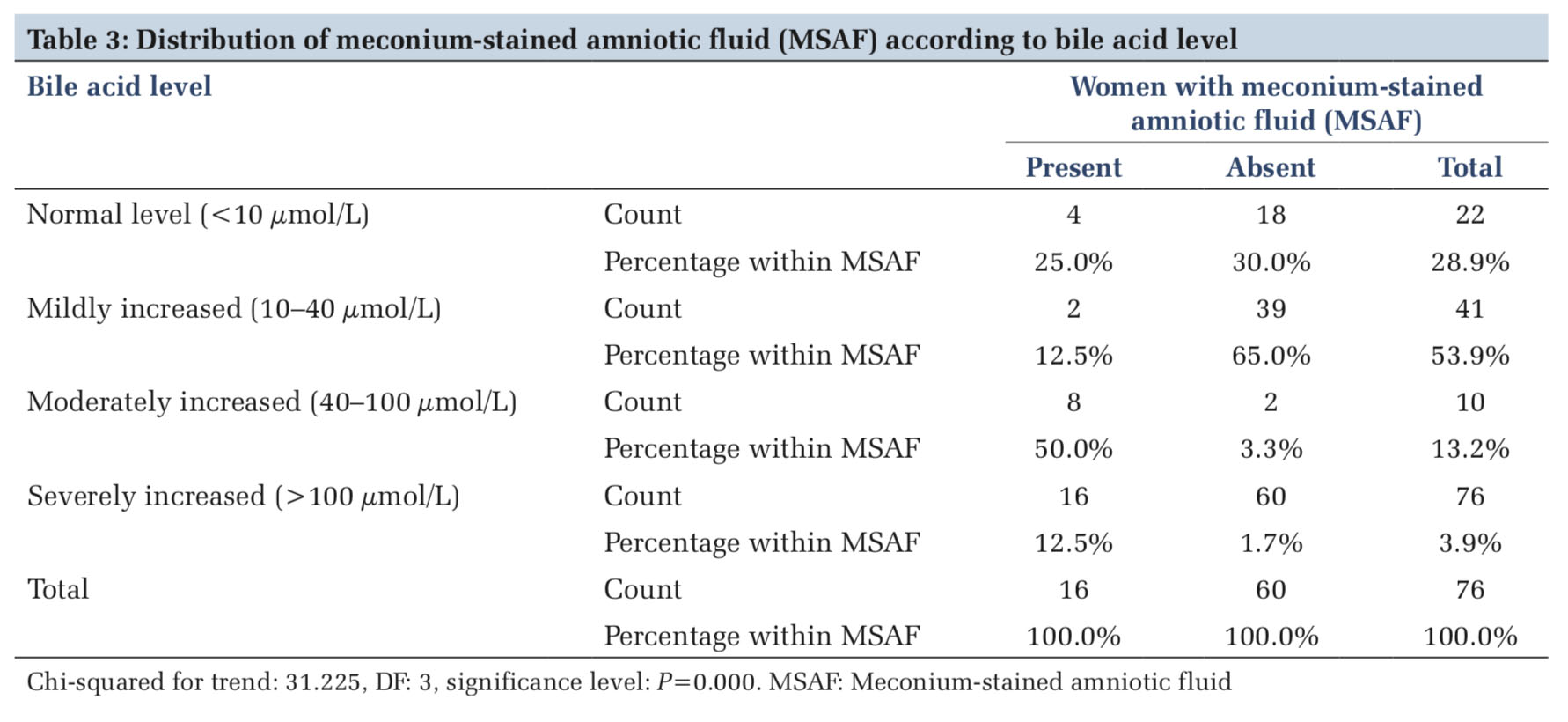
In the present study, distribution of women with MSAF was compared with four groups of women according to bile acid level. MSAF was found in 4 (5.2%), 2 (2.6%), 8 (10.5%), and 2 (2.6%) in the four groups of women categorized as normal (< 10 μmol/L), mild (10–40 μmol/L), moderate (40–100 μmol/L), and severely (>100 μmol/L) increased, respectively. Distribution of MSAF was compared with in different groups of bile acid level by Pearson Chi- squared test to derive p value. The presence of MSAF in comparison to bile acid level was seeming to be highly significant (P < 0.01) (Table 3 and Figure 2).
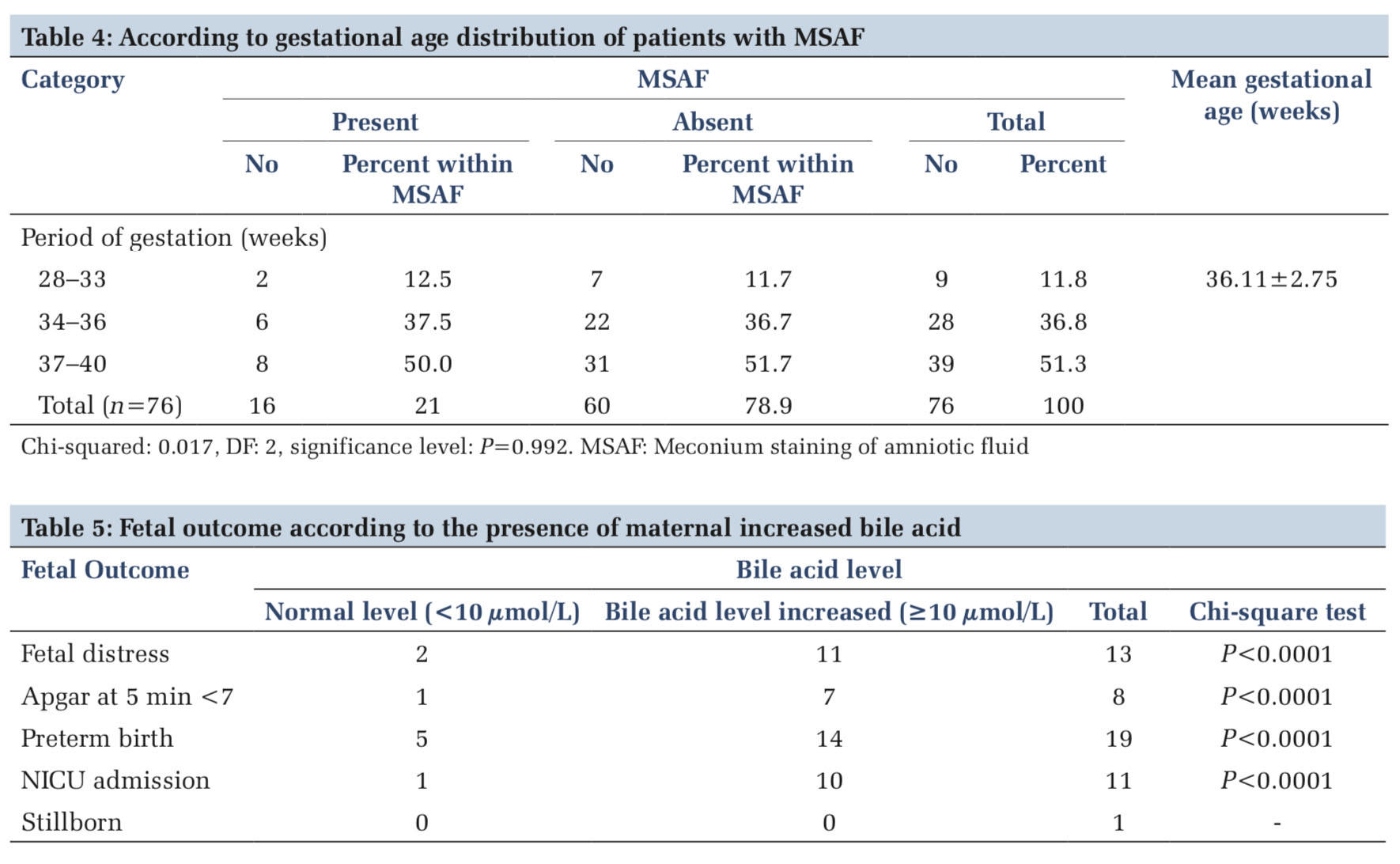
MSAF found in the women with gestational age of 28–33 weeks, 34–36 weeks, and 37–40 weeks, were 2 (2.6%), 6 (7.9%), and 8 (10.5%), respectively. The presence of MSAF was compared with the gestational age and was measured by Pearson Chi- squared test which showed P = 0.992. Period of gestation in comparison to MSAF was not found significant (>0.05) (Table 4).
Pregnancy complicated by fetal distress, Apgar score < 7 at 5 min, admission in NICU, and intrauterine fetal death (IUFD) were found in 13 (17.1%), 8 (10.5%), 11 (14.47%), and 1 (1.31%), respectively. These findings of adverse perinatal outcomes were considered as unexpected outcomes in the study.
Women of elevated serum bile acid level compared with each adverse perinatal outcome and were measured by Pearson Chi-squared test to derive P-value. It was seen that P-value for each adverse perinatal outcome was < 0.01 and seems to be highly significant (Table 5).
Among the women, 33 (43.4%) and 39 (51.3%) were delivered by cesarean section (LUCS) and 4 (5.2%) were by instrumental delivery. PPROM and PPH were found in 6 (7.9%) and 3 (3.9%) women in the study group.
IHC in pregnancy is relatively common in obstetrics practice. Pathogenesis of IHC is thought to be multifactorial, mostly depending on genetic configuration, geographical area, environmental changes, and ethnicity. In the present study, there were 3.3% incidence of IHC found. It is slightly higher than those reported by other authors in similar studies. The studies of IHC were reported by Pegu et al.,[1] Rook et al.,[3] and Pokhrel et al.[4] who were found that 2.73%, 1.9%, and 1.15%, respectively, in their study population.
In the present study, the highest number of IHC affected women was 39 (51.3%) of 21–25 year age.
In another study, the age group of 18–24 years (36%) and 21–25 years (46.67%) was affected most commonly, that was reported by Renu et al.[5] and Mahajan et al.,[6] respectively. These were similar to the finding found in the present study.
Mean age of women was 25.25 ± 3.61 years in the study group. In a similar study, Renu et al.[6] and Celik et al.[13] described that mean age of 26.42 ± 5.79 years and 27.7 ± 5.3 years was found in their study subjects. Their findings were consistent with the finding found in the present study.
Mean gestational age was 36.11 ± 2.75 weeks in the pregnancy which is complicated by IHC within the study group. Mean age of 37 and 38 weeks was reported by Pegu et al.[1] and Arthuis et al.[14] in their similar study.
In our study, MSAF was compared with gestational weeks and was measured by Pearson Chi-squared test, the value of “P” was 0.992. The gestational age in comparison to MSAF was not found significant (>0.05). The studies of IHC reported by Renu et al.[5] were not found in significant association (P > 0.05) of MSAF with weeks of gestational age at delivery in comparison with their control group. Their finding was similar to our study finding, only exception being that we had not adopted any control group.
In the present study, elevated liver enzymes levels were most commonly found but rise level of TB was less common. According to the literature review, the finding of present study was similar with the findings found by Danilidis and Tantanasis[2] and Bhattacharrya et al.[12]
In the present study, 71.1% (54) of women of study subject had elevated serum bile acid level (>10 μmol/L). It was found that increase bile acid level in maternal serum was significantly (P < 0.01) associated with MSAF. In a similar study reported by Geenes et al.,[10] it was found that there was also significant association between increase maternal serum bile acid level and presence of MSAF. In an another study reported by Estiu et al.,[11] it was also found that there was significant association in the presence of MSAF and elevated bile acid level in maternal serum. The findings of the present study were consistent and comparable with the finding.
It was found that fetal distress, low Apgar score at 5th min (< 7), preterm birth (< 37 weeks), admission in NICU, and stillborn were found in 17.1% (13), 10.5% (8), 25% (19), 14.5% (11), and 1.31% (1), respectively. The adverse perinatal outcomes were compared with serum bile acid level and measured by Pearson Chi-squared test and derived P-value was highly significant (< 0.01). Similarly, significant (< 0.01) association of adverse fetal outcome with serum bile acids was found in a study reported by Renu et al.[5]
In the present study, it was also seen that preterm premature rupture of the membranes (PPROM), PPH, cesarean section (LUCS), and vaginal and instrumental delivery were 7.9%, 3.9%, 43.4%, 51.3%, and 5.3%, respectively.
In another study reported by Arthuis et al.[14] on the pregnancy with IHC. In their study, they found emergency and elective caesarean section and vaginal delivery was 13.6%, 12.1% and 74.3% respectively. According to their study, preterm birth, MSF, NICU admission of newborn, and low Apgar score at 5 min (< 7) were 15.7%, 18.6%, 2.9% and 2.9% respectively. All of these findings were consistent with our study findings. Only exception of excessively high PPH rate of 20.7% found in their study, may be due to the different geographical area. PPH rate was excessively high but most of the findings were consistent with the present study findings.
Pokhrel et al.[4] reported in their study that MSAF, fetal distress, low Apgar score at 5th min (< 7), and IUFD were 32.5%, 26.25%, 13.75%, and 6.25%, respectively. In the same study, the author also found that PPROM, PPH, and cesarean section rate (including emergency and elective indications) were 6.25%, 11.25%, and 46.25%, respectively. The study reported by Cui et al.[15] compared the two subgroups of women with IHC of more than and less than serum bile acid level of 40 μmol/L. They found that there was a significant increased risk of adverse fetal outcomes in the group of more than 40 μmol/L of serum bile acid level (Table 6).
In the present study, only one stillborn (1.31%) was delivered by a woman with severely high (>100 μmol/L) bile acid level. The study reported by Li et al.[16] also found 1.2% incidence of stillborn in their study. In the same study, there was increased risk of still birth, found within the women of increased bile acid (>40 μmol/L) level. Their finding was consistent with the findings of the present study.
Due to the retrospective nature of the present study, it has certain limitations and potential for biases. The present study was conducted in a single hospital, to know the effect of increased bile acid level and its potential adverse effect on perinatal outcome, will need larger studies. More research is also required to know the ideal way of fetal monitoring and selection of optimum time regarding delivery of the baby in women.

Women with elevated bile acid are more prone to meconium staining of amniotic fluid and adverse perinatal outcome. Close fetal monitoring and weekly liver enzymes and bile acid assay are needed for deciding optimum delivery time to avoid adverse fetal outcome in pregnancy complicated by IHC.
Subscribe now for latest articles and news.