

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2018.v04i03.002
Year: 2018, Volume: 4, Issue: 3, Pages: 7-12
Original Article
Preeti Rai, Reema Bhushan, Renu Singh
Department of Pathology, Lady Hardinge Medical College, New Delhi, India
Address for correspondence:
Reema Bhushan, 1113, Sector 4, R. K. Puram, Connaught Place – 110 022, New Delhi, India. Phone: +91-9560121204. E-mail: [email protected]
Introduction: The difference in the size of the two ventricles in adult is well known due to the difference in the resistance of systemic and pulmonary circulation. However, the difference between the two during fetal life is rarely studied in India.
Material and methods: In the present study, the normal fetal hearts were selected from 21 to 40 weeks of gestation. The measurement of the right and left ventricular thickness was done using a graduated scale. They were compared with each other and were also compared with body weight, crown-rump length, and head circumference. Total heart weight in these cases were compared with body weight, crown-rump length, head circumference, and gestation age.
Results: The right ventricle showed similar or slightly more dimension than the left ventricle from 22nd week up to 30th week gestation, while there was similar thickness of both the ventricles from 32nd week and beyond.
Conclusion: Linear correlation was obtained between the right ventricular and the left ventricular thickness without much difference in their thickness throughout the gestation.
KEY WORDS:Systemic, pulmonary, dominance.
The ratio of left ventricular (LV) to right ventricular (RV) thickness is described in literature and is approximately 3:1 in adults. The difference between the RV and LV thickness has been reasonably explained due to the difference in the resistance of the two circulations, i.e., systemic and pulmonary circulation. However, very few studies are available which compare them in fetal life, and the information in this field is still limited. The concept of “RV dominance” in the human fetus has been quoted from time to time. This has been supported largely from autopsy studies which showed RV weights to be more than LV weights. However, there was wide variation in the methods used in these studies. The different proportions of the interventricular septum were designated as being the part of the right and left ventricles. Due to this, there were dissimilar growth curves in these studies.[1]
Cardiac growth assessment done using the real-time ultrasound has provided conflicting results. Few studies showed that the right ventricle is larger than left ventricle,[2] while others showed them to be of similar size.[1,3-5] Studies, thus done focusing on the ultrasonographic findings, have also found to have many limitations.[1] Fetal ultrasound studies were performed using Doppler color flow imaging system in many of these studies. M-mode tracing was done using M-line perpendicular to the interventricular septum. This was found to be difficult to perform and understand.[6] Moreover, different proportions of the interventricular septum were arbitrarily designated as the right and left ventricles in these studies, hence resulting in dissimilar, multiphasic ventricular growth curves.[1]
Very few studies are available in the literature on comparing ventricular thickness done by performing the gross anatomy of fetal heart, especially from India. Hence, we attempt in the present study to compare the difference in RV and LV thickness in fetal heart and to find its relation if any, with gestational age, total body weight, heart weight, crown-rump length, and head circumference.
The study was conducted in the Department of Pathology, Lady Hardinge Medical College, NewDelhi. Dead or stillbirth fetus was sent for evaluation of any fetal cause of death. The gestational age of fetus was established from menstrual age. Any macerated fetus or fetus with malformation was excluded from the study. 20 fetal hearts with gestational age ranging from 21 to 40 weeks were dissected and included in the study. The heart was opened by inflow-outflow method. The initial cut was made from the inferior vena cava to the superior vena cava, and the right atrium is inspected. The RV cavity was opened by cutting through the atrioventricular valve and along the outflow tract. This exposed the RV free wall. The left atrium is opened between the right and left upper pulmonary veins. The LV tract is opened along the inferolateral aspect through the left atrial wall, through the midportion of the mitral valve until the apex. Ventricular wall thickness of the left and right side was measured just below the coronary sulcus using a graduated scale.
Comparison of the RV and LV wall thickness was done using Pearson coefficient. The comparison of ventricular wall thickness was also done with gestational age, body weight, heart weight, crown- rump length, and head circumference using linear regression analysis.
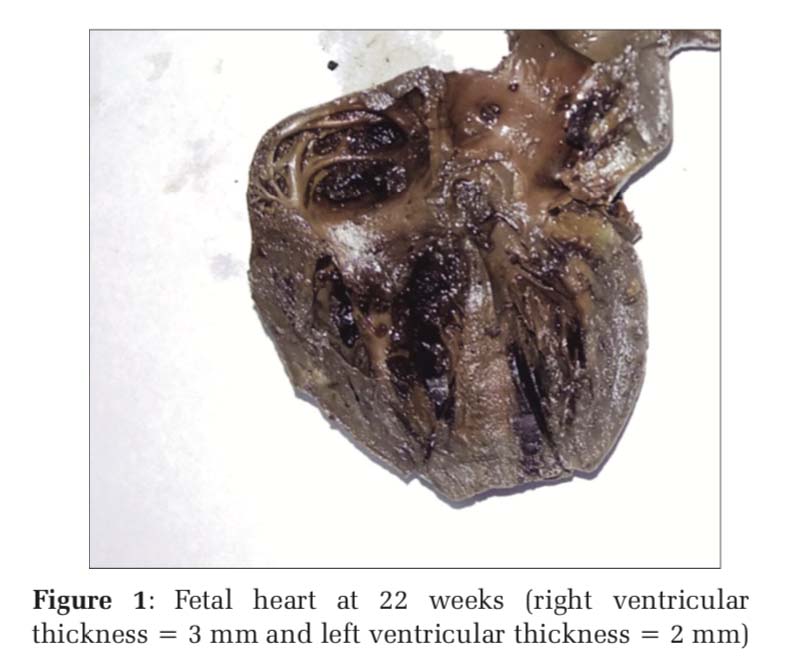
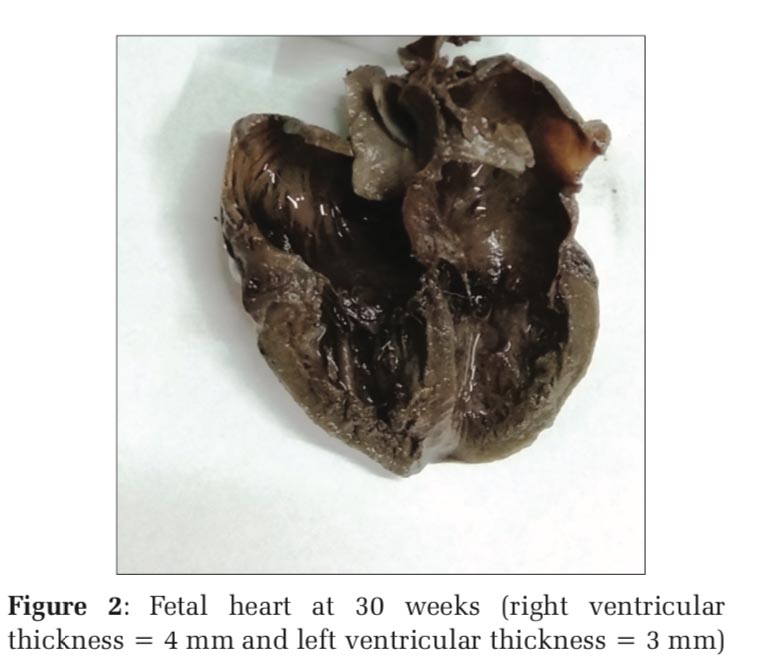
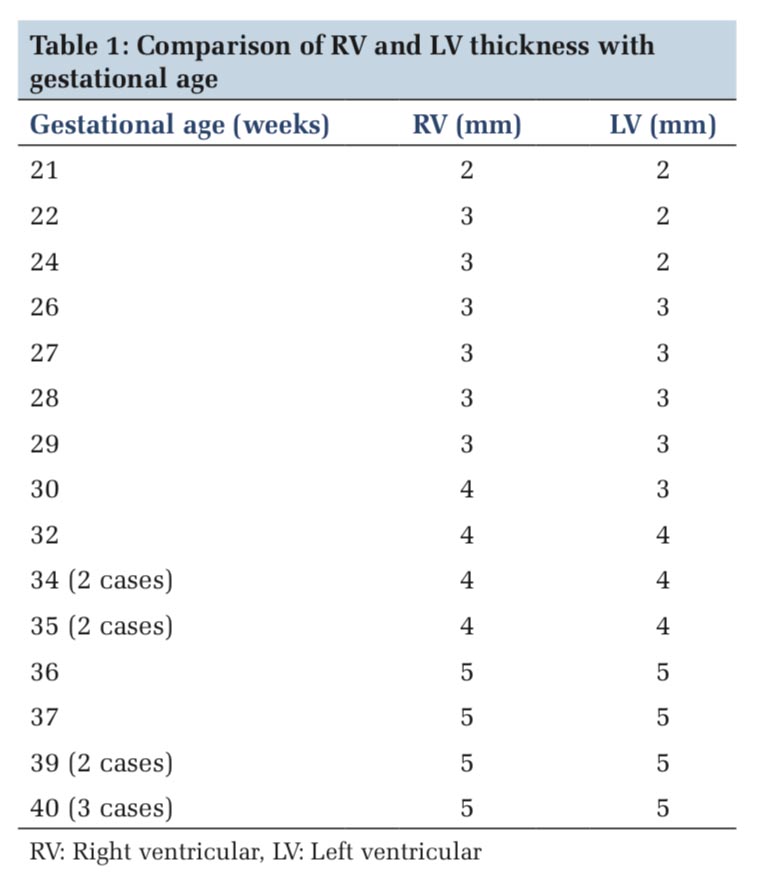
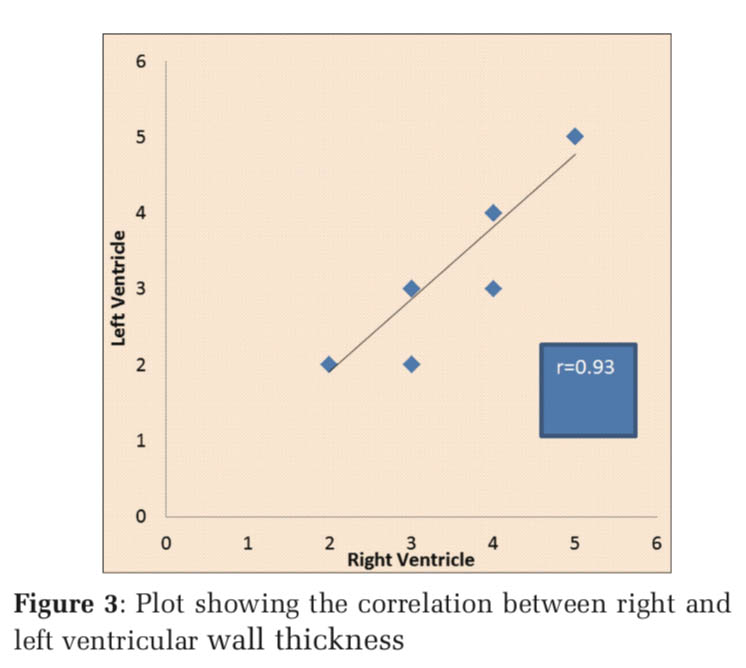
In the earliest age group of 21 weeks, the right ventricle measured similar to the left ventricle. From 22nd week to 30th week gestation, the thickness of the right ventricle showed similar or slightly more dimension than the left ventricle. Figure 1 depicts the fetal heart at 22 weeks of gestation (right ventricular thickness = 3 mm and left ventricular thickness = 2 mm) while Figure 2 depicts the fetal heart at 30 weeks of gestation (right ventricular thickness = 4 mm and left ventricular thickness = 3 mm) included in the study. Measurement of ventricular size from 32nd week and beyond, however, showed similar right ventricle and left ventricle thickness, i.e., 4 mm from 32nd to 35th week and 5 mm from 36th to 40th week (Table 1). The RV wall thickness verses LV wall thickness showed a linear correlation coefficient of 0.93 (P < 0.001)(Figure 3). Thus, on the contrary to the finding in adult heart which shows an increased thickness of the left ventricle compared to the right ventricle, the RV wall thickness is slightly more or similar to the left ventricle during fetal life.
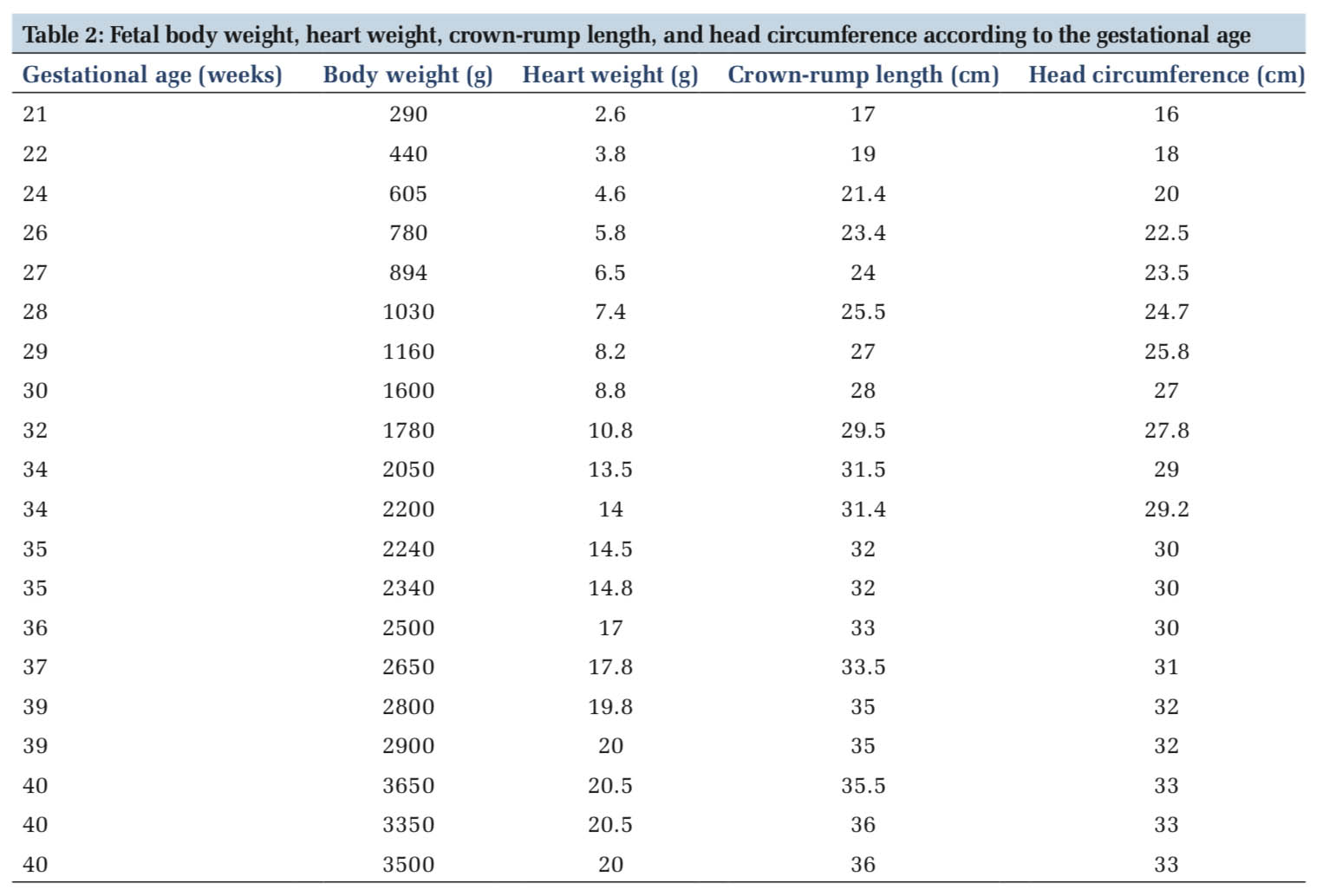
Table 2 compares fetal body weight, heart weight, crown-rump length, and head circumference according to the gestational age. Total heart weight increased about 10 times between 21 weeks to term, from 2.6 g to 20.3 g (mean). RV and LV free wall thicknesses both increased from 2 mm to 5 mm from 21 to 40 weeks’ gestation.
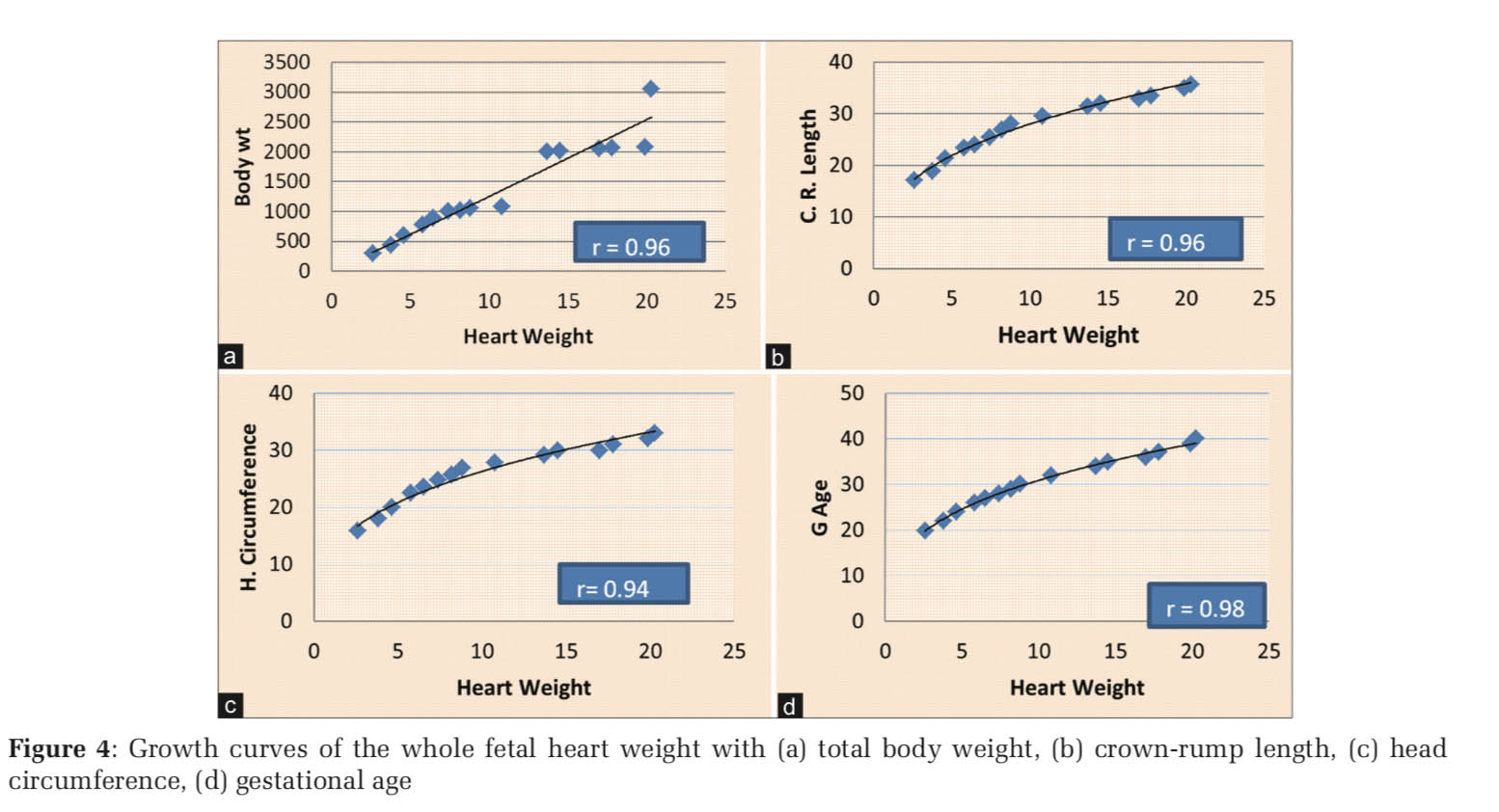
Total heart weight increased linearly with body weight (r = 0.96, Figure 4a), crown-rump length (r = 0.96, Figure 4b), head circumference (r = 0.94, Figure 4c), and gestation age (r =0.98, Figure 4d) (P < 0.001).
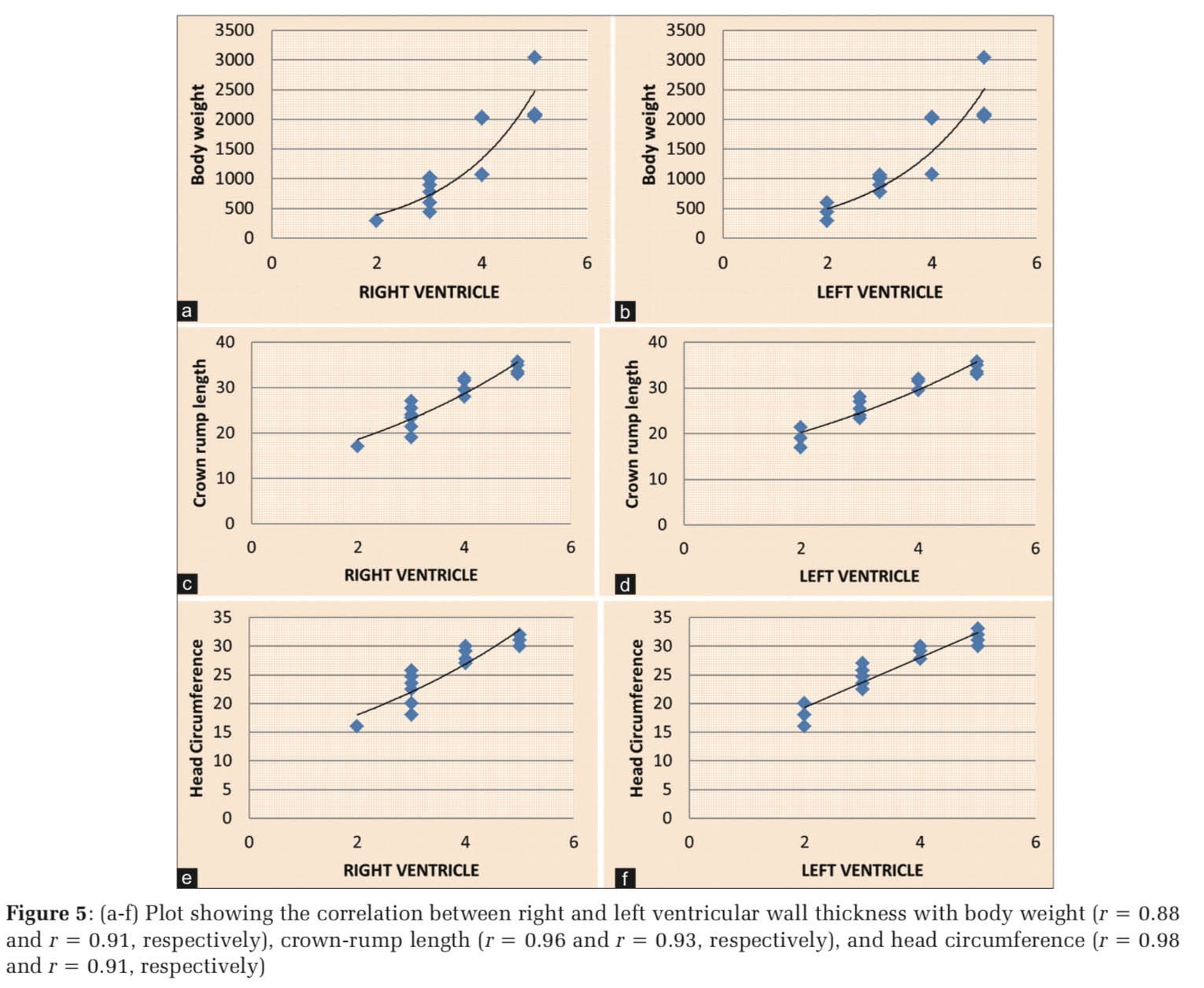
The RV and LV wall thicknesses increased slight exponentially with body weight (r = 0.88; Figure 5a, and r = 0.91, Figure 5b, respectively), while they increase linearly with crown-rump length (r = 0.96, Figure 5c, and r = 0.93, Figure 3d, respectively), and head circumference (r = 0.98, Figure 5e, and r = 0.91, Figure 5f, respectively) (P < 0.001).
Sutton et al.[3] did their study on heart specimen obtained from 55 spontaneously aborted human fetuses from 8 weeks to 40 weeks gestation. The RV and LV free walls were dissected parallel to the planes of the respective atrioventricular valve. RV and LV midwall thicknesses were measured in the wall opposite the middle of the septum, using a binocular microscope. In addition, total heart weight, RV and LV free wall thicknesses, and respective surface areas were also measured. They showed an increased thickness of the RV and LV free wall from 0.13 cm to 0.52 cm and that the thicknesses of both the walls do not differ significantly from each other. Moreover, it also showed a linear correlation with crown-rump length, head circumference, and body weight. In addition, they also observed that the total heart weight increased linearly with body weight but exponentially with crown-rump length, head circumference, and menstrual age. In the present study, RV and LV thickness did not differ significantly and increase linearly with the crown-rump length and head circumference and exponentially with body weight. The total heart weight in the present study increased linearly with body weight, crown-rump length, head circumference, and gestational age. Kim et al. in their study on fetal heart development showed that both the ventricular walls, the interventricular septum, and both ventricular cross-sectional areas showed linear regression, and the left-to-right wall thickness ratios were nearly constant. They concluded that the left-to-right wall thickness ratios were nearly constant throughout the gestation.[7] There were other studies in the past which do not support the RV dominance in the developing human fetal heart.[3,7,8] Oyer et al.,[9] retrospectively, studied 776 cases of fetal and neonatal autopsy from 15 to 42 weeks of gestational age. The RV wall thickness was measured in the area excluding the trabecular muscle in the inflow portion at one-third of the distance from the atrioventricular ring to the apex. They further compared the mean value obtained in their study with the studies done by Tan et al., Firpo et al., and Sharland et al.[10-12] Oyer et al. observed that the LV wall thickness exceeded the RV wall thickness at all ages. However, echocardiography done in their study showed LV:RV wall ratio to be 1 throughout the period of gestation. Mandorla et al. performed the study on the fetal heart using real- time-directed M-mode ultrasound from the 19th week of gestation until term. They stated that the RV and LV chamber size, right and left atrial chamber size, interventricular septum thickness, RV and LV wall thickness, and aortic and pulmonary valve diameter all increased linearly with age. They also quoted that the right ventricle was slightly larger than the left ventricle (P < 0.001).[13]
Studies have quoted that the similarities between the right and left ventricles may be explained by their respective functions. During fetal life, oxygenated blood from the placenta goes into the left atrium through a patent foramen ovale. This blood flow depicts the preload to the ventricle. Due to patent foramen ovale, preload in the two ventricles must be the same. The right ventricle ejects > 95% of its stroke volume into ductus arteriosus. It has comparatively similar diameter to the pulmonary trunk and ascending aorta. Thus, both the ventricles effectively eject into a common systemic circulation. Thus, the right and left ventricles are having same preload and similar after loads during the fetal life. As afterload also plays a role in determining ventricular mass and thickness, hence RV and LV muscle masses and thickness can be the same.[3,14,15]
The study has a limitation that the number of fetal hearts included was twenty in number. However, it emphasizes the need for more studies including a large number of cases that will be more authentic to give definitive conclusion.
We conclude that linear correlation was obtained between RV and LV thickness and that they do not differ much throughout the gestation. The RV wall thickness is slightly more or similar to the left ventricle during fetal life contrary to the finding in adult heart. Heart weight also shows a linear correlation with parameters such as body weight, crown-rump length, head circumference, and menstrual age.
Subscribe now for latest articles and news.