

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2019.v05i02.002
Year: 2019, Volume: 5, Issue: 2, Pages: 9-13
Original Article
Jyotsana Bharshankar, Archana Mandape
Department of Physiology, Government Medical College, Chandrapur, Maharashtra, India
Address for correspondence:
Dr. Archana Mandape, Department of Physiology, Government Medical College, Chandrapur, Maharashtra, India.
E-mail: [email protected]
Introduction: Increased parasympathetic drive in asthmatics is observed by many researchers. However, some have found increased sympathetic activity and others found it decreased.
Materials and Methods: A case–control study was planned including 30 asthmatic patients and 30 age-matched non-asthmatics as controls. Spirometry and autonomic functions were tested, and results of the mean difference were compared using the Student t-test.
Results: Out of 30 asthmatics, 76% were in mild-to-moderate grade of asthma on the basis of their forced expiratory volume in the first second % values. Rise in diastolic blood pressure with cold pressor test was significantly less in asthmatics than controls (5.66 ± 3.415 and 11.73 ± 4.49 mmHg; P < 0.001). The mean Valsalva ratio in asthmatics was significantly less than in controls (1.158 ± 0.116 and 1.418 ± 0.645; P < 0.05). Galvanic skin resistance in asthmatics was significantly more than in controls (589.8 ± 268.12 and 328.75 ± 165.07; P < 0.001).
Conclusions: Thus, from our study, there was increased parasympathetic and decreased sympathetic drive in patients with bronchial asthma as compared to controls.
KEY WORDS:Autonomic function tests, bronchial asthma, spirometry.
The volume of respiratory apparatus is governed by various factors such as – tone of the bronchial smooth muscles, functions of the epithelial cells, secretions like mucus, blood flow in the interstitium, permeability in the microvessels, and secretions of certain inflammatory mediators. All these factors are controlled by autonomic nervous system. This system operates reflex through the neural circuits or pathways which are adrenergic, cholinergic, and non-adrenergic non-cholinergic in nature.[1] Asthma and its associated inflammation in the airway affects functions of the primary afferent, integration in the central nervous system, synaptic transmission in autonomic ganglia, and the transmission at the postganglionic neuroeffector junction.[2]
Dysfunctions in the autonomic nerves of airways which regulate the smooth muscles account directly or indirectly for many symptoms and clinical features of asthma.[3] Enhanced cholinergic influence to the airways has been established as a contributing factor to the development of bronchial asthma.[4,5] Beta-adrenergic blockade develops severe bronchoconstriction in asthmatic patients, whereas in normal people, no significant effect was observed on the airway caliber.[6] The mechanism behind this is not yet identified. The role of adrenaline in the control of airway caliber in asthma is uncertain.[7] Some studies also report sympathetic hyperactivity in asthmatic patients to combat the brochoconstriction.[7,8]
The cardiovascular and respiratory autonomic efferent nerves have common central origin.[9] Therefore, alteration in the cardiovascular responses may reflect the abnormality in autonomic nervous system. From the literature review, we hypothesize that there are some or other kind of autonomic disturbances in asthma patients. On this principle, the non-invasive, safe, and easily reproducible, autonomic function tests have been used to observe the alterations in autonomic functions in asthmatic patients.
A hospital-based cross-sectional study was conducted in thirty diagnosed asthmatic patients who were selected as cases and equal number of non-asthmatic persons served as controls. The study participants were equal number of males as well as females aged between 25 and 46 years. Sample size was calculated using the formula for difference of mean, power of the study was 80%, α-error was 1.96, and significance level was set at 0.05.
Thirty known asthmatic patients having history and clinical features of asthma were recruited from the asthma clinic of our Institution. Duration of asthma was more than 5 years with at least two asthmatic exacerbations in any year. Patients with asthmatic attack within 2 weeks of study and those taking medications that may alter autonomic functions were excluded. Oral theophylline and β2 adrenergic agents were withheld for at least 8 h before the investigations. Thirty normal volunteers in the same age group were selected from the non-teaching staff members of our institution and the general population as controls.
Those suffering from diabetes, hypertension, ischemic heart disease, arrhythmia, liver disease, chronic bronchitis, tuberculosis, hypothyroidism, and peripheral nerve diseases were excluded from the study. Individuals having personal habits of alcoholism, tobacco smoking or chewing and addiction to any drugs, and pregnant women were excluded. The study protocol was approved by the Institutional Ethics Committee, and informed written consent was obtained from all the study participants.
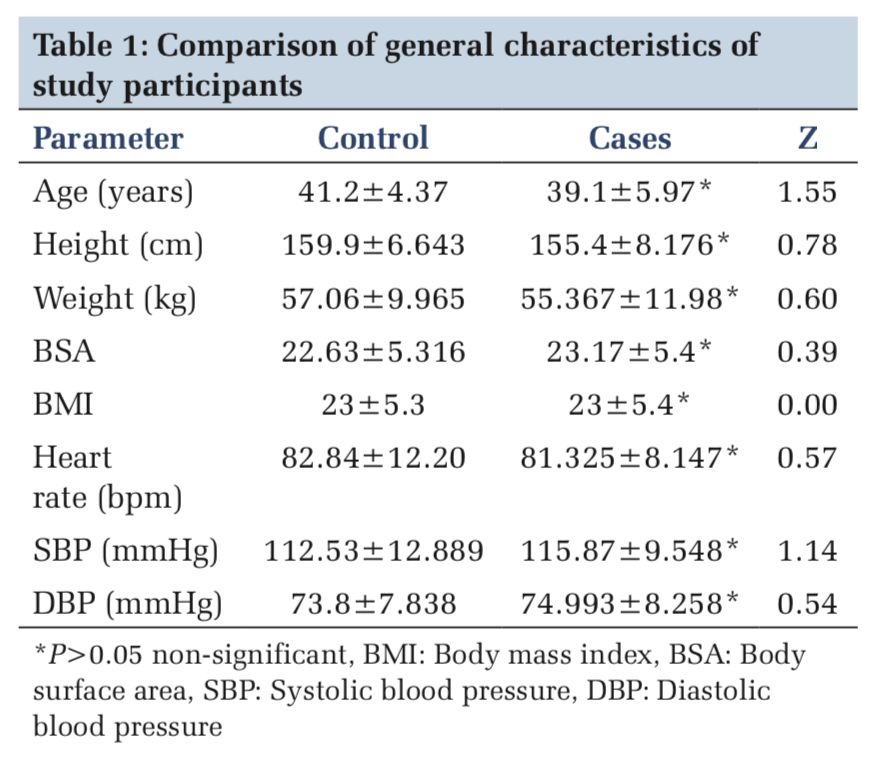
The general demographic profile of each study participant was taken, and the appointment was given with proper instructions to assess the pulmonary function and autonomic function tests in the department of physiology. The values for the parameters such as forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF) were obtained using Medspiror (JK medical system Pvt. Limited., Chennai) in standing position. Severity of asthma is graded, based on FEV1% of predicted value according to guidelines given by the global initiative for asthma updated 2008. FEV1 ≤60% is graded as severe, FEV1 60–80% as moderate, and FEV1 ≥80% is graded as mild asthma.[10]
Autonomic function tests were done for sympathetic and parasympathetic divisions using 16 channel computerized polygraph machine (Physiopac, PP-4, Medicaid system, Chandigarh, India). Other instruments used were hand dynamometer, laboratory thermometer, 40mm U-tube mercury manometer.
Cold pressor test (CPT)
The procedure was explained, and resting blood pressure (BP) was recorded. The participant sat in comfortable position and immersed his/her hand in cold water maintained at 4–6°C. BP was recorded from the other sided hand every 30 s, and maximum rise in diastolic BP was noted.[11]
Handgrip test (HGT)
The BP response to isometric exercise was measured as the participant was asked to hold the hand dynamometer in the dominating hand and compress the hand with 30% of the maximum effort. The BP was recorded from the other sided hand every 30 s and rise in diastolic BP just before releasing the handgrip was recorded.[12]
Galvanic skin resistance (GSR)
It was recorded using two silver chloride disc electrodes filled with electrode jelly placed at 4 cm apart on the palmer surface of right hand. GSR values were sampled continuously at 20 s interval. The value after 1 min was noted which was calculated automatically by the computerized polygraph.[13]
30:15 RR ratio
Sphygmomanometer and electrocardiography (ECG) leads were attached to the participant lying in the supine position. BP and ECG in lead II were recorded in resting and also when the participant stands up immediately. Longest RR interval about 30 beats after standing divided by shortest RR interval about 15 beats after standing was calculated as 30:15 RR ratio.[14] Standing/lying ratio: Participant stood quietly for few minutes with ECG leads attached then lie down immediately without support. Continuous ECG was recorded from 20 beats before lying down and 60 beats after lying down. Procedure was repeated at the interval of 5 minutes, average RR during 5 beats before lying down and shortest RR interval during 10 beats after lying down was noted, and ratio was calculated.[14] Valsalva ratio: The Valsalva maneuver was explained to the participants. ECG in lead II was attached and was asked to strain by blowing against closed glottis into the mouthpiece attached to the mercury manometer and maintain the pressure of 40 mmHg for 15 s. A continuous ECG record was obtained 1 min before strain during the straining phase and 45 s following the strain release. The ratio of the longest RR interval after strain to the shortest RR interval during strain was then calculated.[15]
Statistical analysis
The mean values for pulmonary function tests and autonomic function tests were obtained in both the groups. The values of all the parameters were subjected to student t-test for testing the significant difference in the mean. P < 0.05 was considered statistically significant.
The mean value of age in control group was 41.2 ± 4.37 years, and in asthmatic patients, it was 39.1 ± 5.97 years. The difference in the mean was statistically not significant. The height, weight, and body surface area showed no significant difference in the mean values (Table 1).
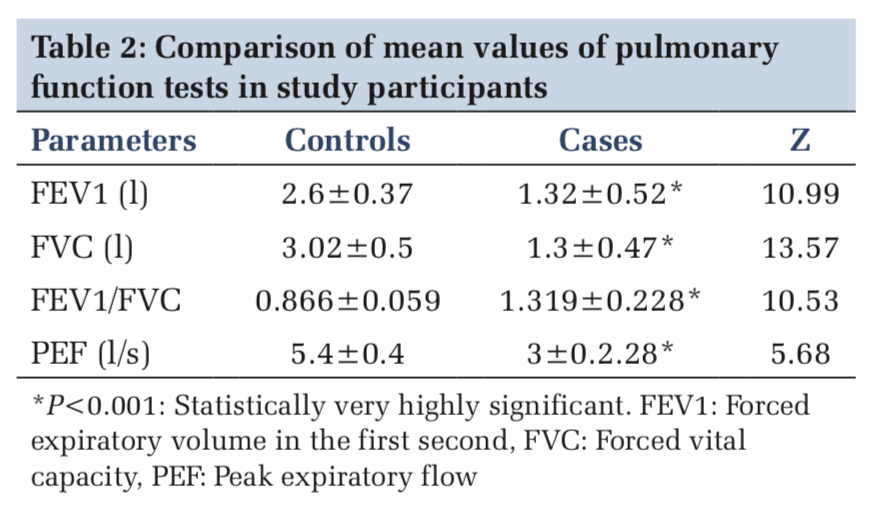
The mean values of pulmonary function tests such as FEV1, FVC, FEV1/FVC ratio, and PEF were tested for significant difference. FVC was 3.02 ± 0.5 l in controls and 1.3 ± 0.47 l in cases. FEV1 was 2.6 ± 0.37 l in controls and 1.32 ± 0.52 l in cases. PEF was 5.4 ± 0.4 l/s in controls and 3 ± 2.28 l/s in cases (P < 0.001) (Table 2).
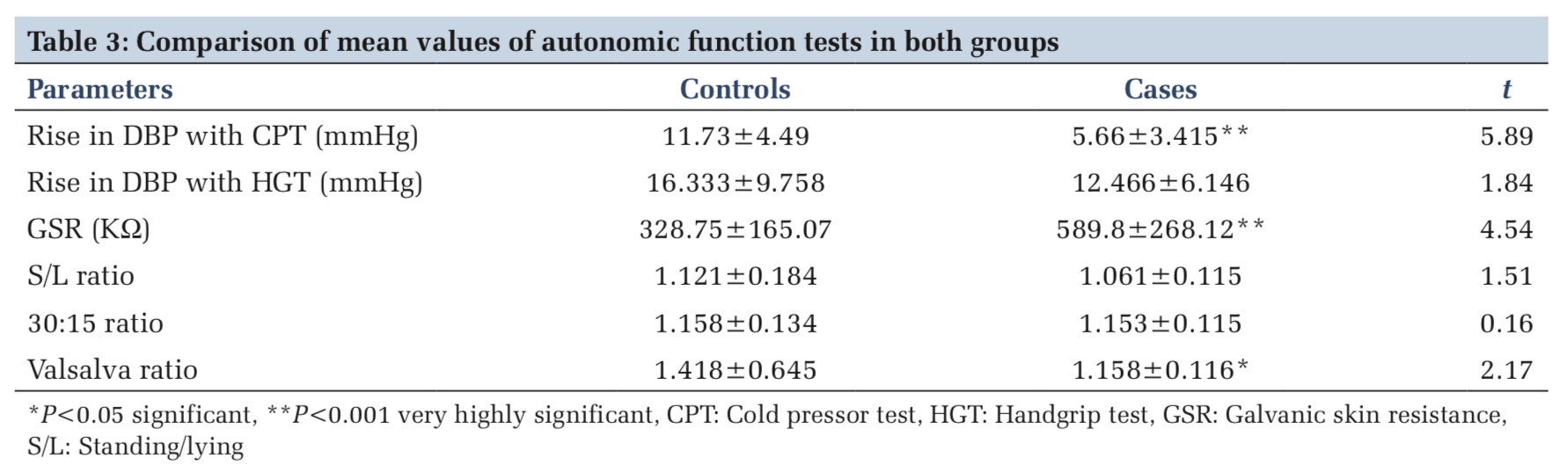
Rise in diastolic BP with CPT in control group was 11.73 ± 4.49 mmHg and it was 5.66 ± 3.41 mmHg in cases (P < 0.001). GSR in asthmatics was 589.8 ± 268.12 and it was 328.75 ± 165.07 in controls (P < 0.001). The standing/lying ratio was 1.2 ± 0.18 in controls and 1.061 ± 0.115 in cases (P > 0.05). 30:15 ratio was also slightly lower in cases than controls but statistically not significant. The Valsalva ratio was 1.158 ± 0.116 in cases and 1.418 ± 0.645 in controls (P < 0.05) (Table 3).
Cardiovascular and respiratory regulatory mechanisms are governed by autonomic nervous reflex system. In respiratory system, parasympathetic innervation is abundant in larger airways and diminishes toward smaller airways. Sympathetic innervations to airway smooth muscles are sparse, but glands and blood vessels are heavily innervated by it. Apart from these, there are noradrenergic norcholinergic stimulatory and noradrenergic norcholinergic inhibitory fibers. Parasympathetic stimulation through the vagus nerve is responsible for the slightly constricted smooth muscle tone in the normal resting lung.[16] Therefore, testing the dysfunctions in various conditions, the autonomic function tests which challenge these reflexes are simple, non-invasive, reproducible, and standard tests which are helpful in evaluating the functional status of autonomic nervous system.
As per the results of our study, resting heart rate was lower in asthmatic individuals than in controls although not significant. It suggests increase in the parasympathetic drive in asthmatic individuals. The resting BP, especially the diastolic BP, was on higher side in asthmatic patients. This may be because of the increased α-adrenergic drive in asthmatic patients as quoted by other workers who got similar findings.[1,17] Some authors got resting tachycardia in asthmatic patients.[8,18]
Rise in diastolic BP with CPT was significantly less in asthmatic individuals as compared to controls. The rise in DBP on sustained handgrip was less in asthmatics than controls but was not significant. The decreased response to the challenges shows abnormality in the reflex autonomic activities.[1,8] Mitkari et al.[17] found that the rise in diastolic BP after CPT and HGT was significantly less in asthmatics as compared to controls which is similar to our results. Kumar et al.[1] found that rise in DBP after CPT and HGT was higher in asthmatics than controls, and Shah et al.[8] reported no significant rise observed in DBP with HGT and CPT. These results although in contrast with ours show autonomic dysfunction in asthma patients. The GSR showed significantly higher values in asthmatic patients. This may be because of the decreased activity of sympathetic cholinergic fibers to the skin.[19]
The Valsalva ratio which means the baroreflex sensitivity is significantly decreased in asthmatic patients. This suggests that the parasympathetic hyperactivity in them has blunted the response to the Valsalva maneuver.[8,17] The cardiovascular and respiratory autonomic efferent nerves have common central origin. The standing/lying ratio and 30:15 ratio was less in asthmatics as compared to controls but the difference was not statistically significant. Mitkari et al.[17] and Kallenbach et al.[5] observed significantly low 30:15 ratio in asthmatic individuals as compared to controls.
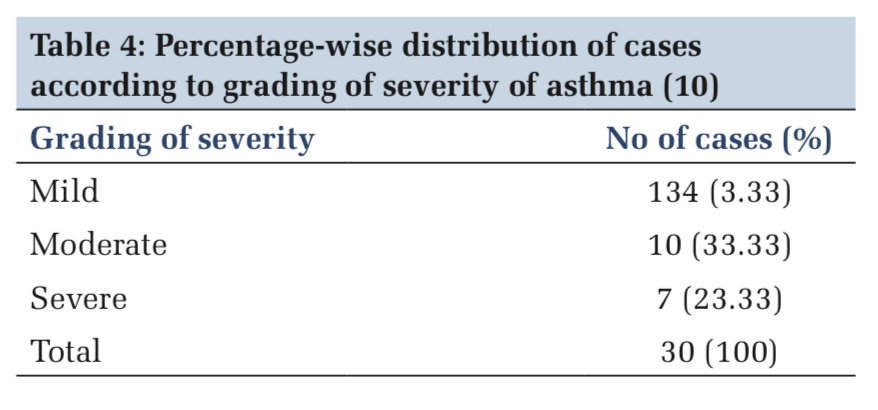
Mourya et al. studied the autonomic functions in asthma patients using seven non-invasive tests. The duration of asthma varied from 8 months to 40 years. He found that out of 45 patients, 33 patients had abnormal autonomic function tests of which 21 patients had only parasympathetic dysfunction, 12 had both sympathetic and parasympathetic dysfunction, and only 6 had isolated sympathetic dysfunction.[20] Devi et al. found increased sympathetic activity in asthma patients, and there was a positive correlation between the severity of the disease and sympathetic dysfunction.[21] Borse et al. found parasympathetic hyperresponsiveness in asthma patients with autonomic function tests.[22] Vagus nerve includes afferent fibers from sensory receptors in the bronchial epithelium. On the basis of studies in animals, it is thought that stimulation of these receptors by irritants can initiate a reflex impulse along vagal efferents that cause release of acetylcholine and bronchoconstriction. Parasympathomimetic drugs cause bronchoconstriction in asthma patients. Parasympatholytic drugs cause bronchial dilatation in asthma patients as well as in normal individuals.[23] The autonomic function test which we used to study the parasympathetic division also showed increased parasympathetic response in asthma patients. Sympathetic abnormalities are more in patients with severe asthma. Mild- to-moderate asthmatics also show sympathetic abnormalities but to a lesser extent than severe asthma.[19] As our maximum patients (76%) fall in the category of mild-to-moderate asthma (Table 4), this could be the possible explanation for decreased sympathetic activity in asthmatic individuals in our study.
Thus, from our study, we conclude that there is a definite alteration in the autonomic nervous responses in asthmatic patients. Both sympathetic and parasympathetic components are affected to different degrees. However, the longitudinal studies with more sample size and supporting biochemical investigations could possibly give more definite conclusions.
We are thankful to the participants for their cooperation during the study.
Subscribe now for latest articles and news.