Introduction
Globally breast carcinoma (BC) is the commonest malignancy among females. 1, 2 In India, BC accounts for 13.5% of all cancers and 10.6% of all cancer associated mortality and many studies have shown a significant increasing in its incidence. 1, 2 BC is a complex disease with many histological subtypes exhibiting varied biological characteristics and prognosis. 3, 4 Further, BC’s of similar histomorphological subtype may exhibit differing behavior and treatment outcomes. 3, 4 Thus the traditional classification based on histomorphology may not completely reflect the biological and behavioral tumor characteristics and is insufficient for patient tailored targeted therapy. 3, 4 In order to overcome these limitations of histological classification, molecular classification based on gene expression profiling, hormone receptor status and immunohistochemical findings was developed. 5 The latter classification comprised of five subtypes, each with distinct gene expression pattern, clinicobiological features and treatment response and outcome: Luminal A, Luminal B, HER2-Enriched, basal like and normal breast-like. 5, 6, 7, 8 Molecular subtyping of BC may provide substantial information required for formulating patient -specific therapeutic strategies and improves risk stratification by identifying patients who may really benefit from neoadjuvant chemotherapy outweighing its toxicity. 5
However, the molecular classification has drawbacks with respect of analytical standardization and utility in different populations. 7, 8 We attempt to classify BC into molecular subtype using immunohistochemistry and to evaluate its association with certain histopathological prognostic factors.
Materials and Methods
This Unicentre cross-sectional study was conducted on resected BC specimens with axillary lymph node clearance, received in the Department of Pathology M.S. Ramaiah Medical College and Hospitals, Bengaluru, over a duration of 2 years during the period from October 2019 to September 2021, after obtaining the institutional ethical clearance.
Cases with only limited surgery without lymph node clearance and cases with extensive tumor necrosis without sufficient viable tumor cells for immunohistochemical evaluation were excluded.
The resected specimens were received in 10% formalin. Detailed gross examination was done and the standard protocol for surgical grossing of oncology specimens was followed. Multiple tissue blocks were taken from tumor, surgical margins and all lymph nodes. These were processed as per standard protocol and paraffin embedded blocks were cut and stained by hematoxylin and eosin (H & E). The H & E stained sections were studied for the tumor histology, grade, lymph node metastasis and other features as per CAP protocol. The BC’s were staged according to American Joint Committee on Cancer (8th edition-2017) staging system.
Immunohistochemistry (IHC): Immunohistochemical detection of ER, PR, Her2neu and Ki- 67 was done in all cases , on tumor sections, using “DAKO REALTM Envision TM detection system” with primary mouse monoclonal antibodies against ER (cloneEP1 DAKO), PR (clonePgR636 DAKO), Her2neu (cloneCB11 BIOGENEX) and Ki67 (clone MIB-1) proteins. Allred scoring system was used for ER and PR reporting and a score of 0 - 2 was taken as negative whereas a score of 3 - 8 was taken as positive. ASCO/CAP guidelines were followed for Her2neu reporting and 3+ staining (uniform intense membrane staining of >10% tumor cells) was considered as positive, 0 - 1+ staining as negative and 2+ as equivocal. Ki- 67 index was calculated as percentage, counting the Ki67 labelled tumor cells out of 1000 tumor cells. Ki-67 labelling index of ≥ 15% was considered as the cut-off point.
Statistical analysis
Data was analyzed using MS Excel SPSS 22 version software (IBM SPSS Statistics, Somers NY, USA). Categorical data was represented in the form of frequency and percentage. Continuous data was represented as mean and standard deviation. Chi-square test or Fischer’s exact test was used as test of significance for qualitative data.
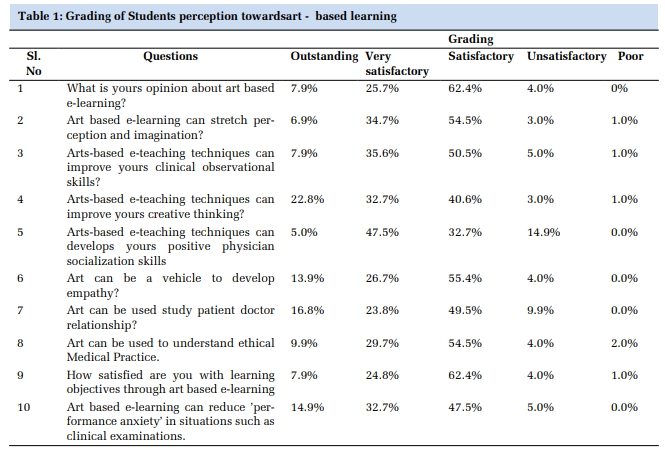
Results
The present study included 85 cases of BC’s comprising 44 (51.8%) modified radical mastectomies, 27 (31.8%) mastectomies with axillary lymph node clearance and 14 (16.5%) wide local excision with axillary lymph node clearance. The mean age at presentation was 51.6±11.9 years (range: 28 - 85 years). The mean tumor size was 4.1±1.8 cm with majority (70.6%) ranging in size from 2 to 5 cm. The commonest histological type discerned was invasive ductal carcinoma (85.9%). A single case of mixed carcinoma comprised of 12% invasive ductal carcinoma, 70% invasive cribriform and 18% papillary carcinoma was diagnosed. Majority of the tumors were of Scarff-Bloom-Richardson (SBR) grade II (moderately differentiated) (51.8%) (Table 1). The mean number of lymph nodes dissected were 16±7 (range: 7 - 33) and nodal metastasis was detected in 65.9% of cases (Table 2). Majority of patients presented in TNM stage II.
|
Characteristics |
All cases |
Luminal A |
Luminal B |
HER-2 |
Basal like |
P value* |
|
|
n= 85 (%) |
n= 43 (%) |
n= 10 (%) |
enriched n= 9 (%) |
n= 23 (%) |
|||
|
Age (years) |
≤ 50 |
51 (60) |
23 (53.5) |
6 (60) |
6 (66.7) |
16 (69.6) |
0.614 |
|
> 50 |
34 (40) |
20 (46.5) |
4 (40) |
3 (33.3) |
7 (30.4) |
||
|
Tumor size (cm) |
≤ 2 |
4 (4.7) |
2 (4.6) |
0 |
0 |
2 (8.7) |
0.892 |
|
2-5 |
60 (70.6) |
30 (69.8) |
8 (80) |
6 (66.7) |
16 (69.6) |
||
|
>5 |
21 (24.7) |
11 (25.6) |
2 (20) |
3 (33.3) |
5 (21.7) |
||
|
Histologic type |
Invasive ductal carcinoma |
73 (85.9) |
35 (81.4) |
8 (80) |
9 (100) |
21 (91.3) |
|
|
Mucinous carcinoma |
6 (7.1) |
5 (11.6) |
1 (10) |
0 |
0 |
||
|
Invasive lobular carcinoma |
2 (2.4) |
2 (4.7) |
0 |
0 |
0 |
||
|
Medullary carcinoma |
2 (2.4) |
0 |
0 |
0 |
2 (8.7) |
||
|
Invasive micropapillary carcinoma |
1 (1.1) |
0 |
1 (10) |
0 |
0 |
||
|
Mixed carcinoma |
1 (1.1) |
1 (2.3) |
0 |
0 |
0 |
||
|
Tumor grade (SBR) |
Grade 1 |
24 (28.2) |
19 (44.2) |
3 (30) |
1 (11.1) |
1 (4.3) |
0.004 |
|
Grade 2 |
44 (51.8) |
21 (48.8) |
4 (40) |
7 (77.8) |
12 (52.2) |
||
|
Grade 3 |
17 (20) |
3 (7) |
3 (10) |
1 (11.1) |
10 (43.5) |
||
SBR: Scarff-Bloom-Richardson grade.
* Statistical test applied: Chi-square test or Fischer’s exact test; Level of significance: P<0.05
|
Characteristics |
All cases |
Luminal A |
Luminal B |
HER-2 |
Basal like |
P value* |
|
|
n= 85 (%) |
n= 43 (%) |
n= 10 (%) |
enriched n= 9 (%) |
n= 23 (%) |
|||
|
Pathological T stage |
T1 |
5 (5.9) |
3 (7.0) |
0 |
0 |
2 (8.7) |
0.855 |
|
T2 |
57 (67.0) |
27 (62.8) |
8 (80) |
6 (66.7) |
16 (69.6) |
||
|
T3 |
18 (21.2) |
9 (20.9) |
2 (20) |
3 (33.3) |
4 (17.4) |
||
|
T4 |
5 (5.9) |
4 (9.3) |
0 |
0 |
1 (4.3) |
||
|
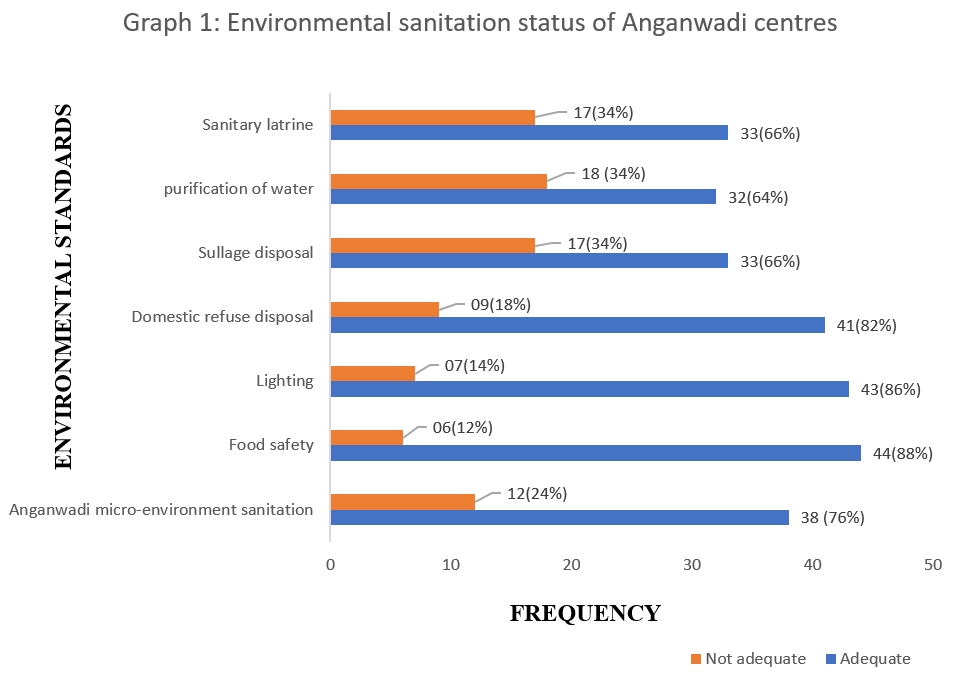
Lymph node metastasis |
Negative (N0) |
29 (34.1) |
20 (46.5) |
3 (30) |
2 (22.2) |
4 (17.4) |
0.139 |
|
N1 |
35 (41.2) |
13 (30.2) |
4 (40) |
6 (66.7) |
12 (52.2) |
||
|
N2 |
12 |
6 (14) |
1 (10) |
1 (11.1) |
4 (17.4) |
||
|
|
-14.1 |
|
|
|
|
||
|
N3 |
9 (10.6) |
4 (9.3) |
2 (20) |
0 |
3 (13) |
||
|
TNM stage |
I |
4 (4.7) |
3 (7.0) |
0 |
0 |
1 (4.3) |
0.78 |
|
II |
51 (60) |
25 (58.1) |
7 (70) |
7 (77.8) |
12 (52.2) |
||
|
III |
30 (35.3) |
15 (34.9) |
3 (30) |
2 (22.2) |
10 (43.5) |
||
* Statistical test applied: Chi-square test or Fischer’s exact test; Level of significance: P<0.05
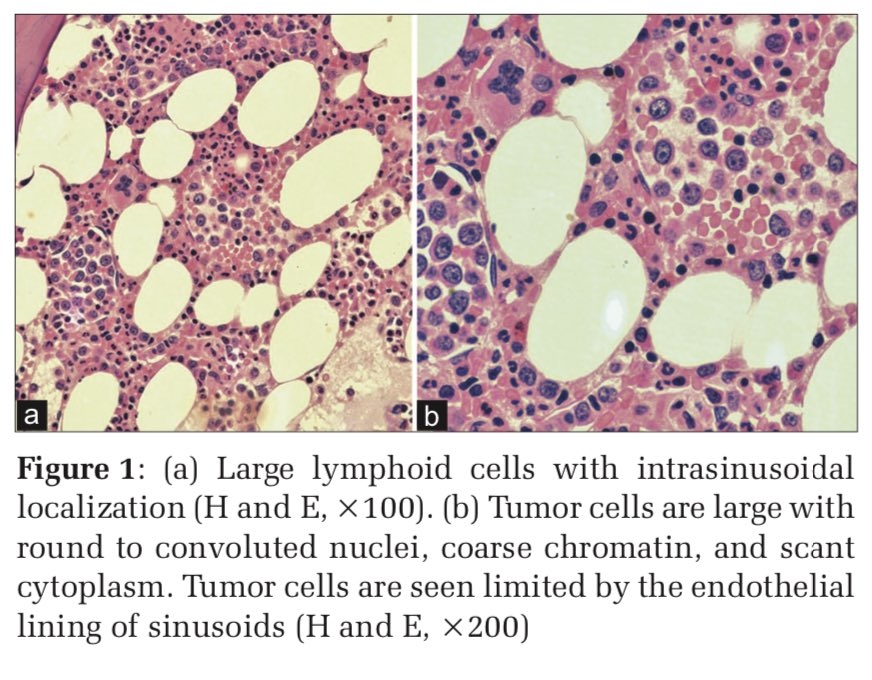
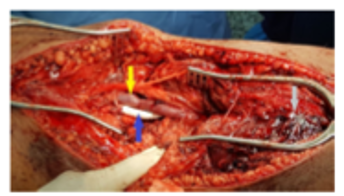
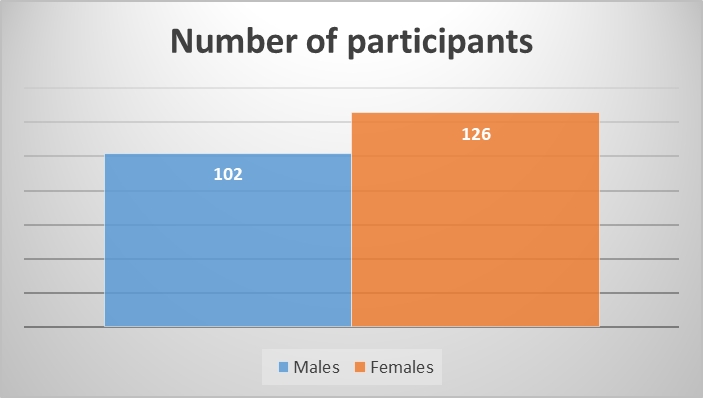
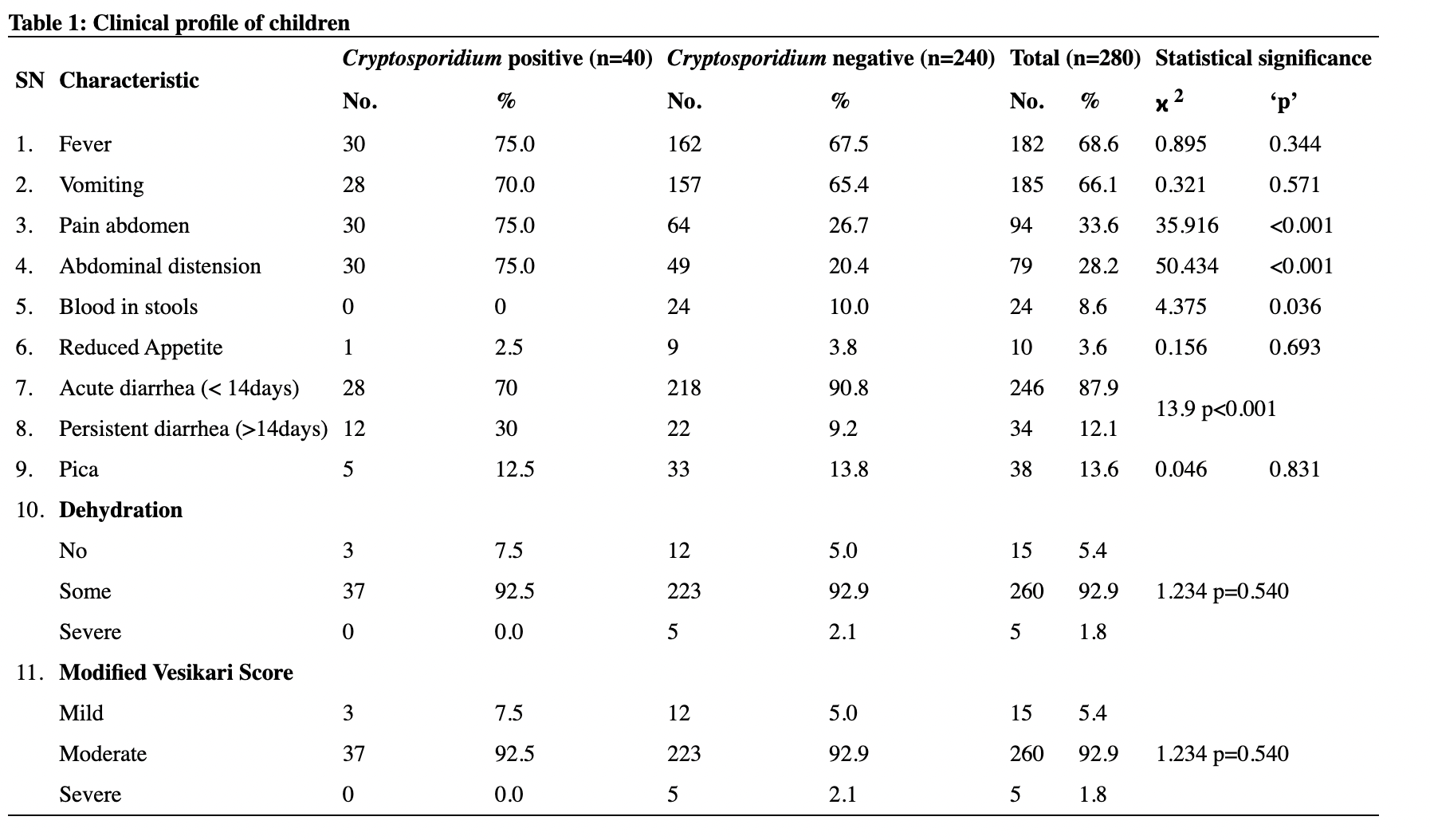
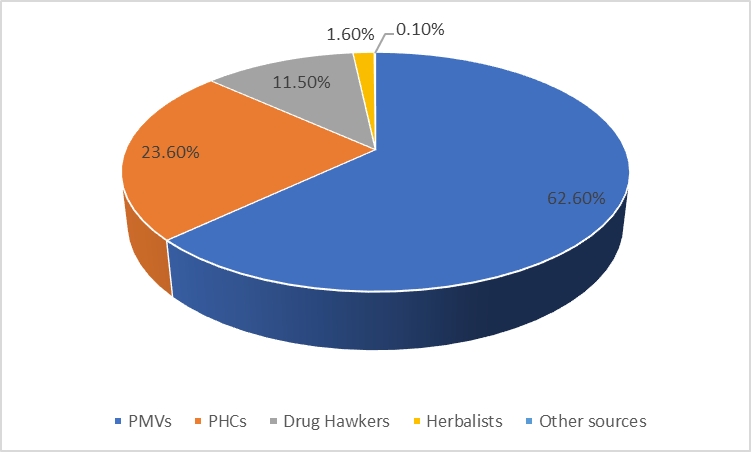
The commonest molecular subtype, was Luminal A (50.6%), followed by Basal like (27%), Luminal B (11.8%) and HER-2 enriched (10.6%). Luminal A tumors showed strong ER/PR expression and were HER2 negative with low proliferative index (Ki-67 index < 15%) (Figure 1). Luminal B tumors exhibited low to intermediate ER/PR and variable HER2 positivity with high proliferative index (Ki-67 index ≥ 15%) (Figure 2). HER-2 enriched tumors were characterized by HER2 positivity, high proliferative index and negative ER/PR expression (Figure 3). Basal like tumors were negative for ER/PR and HER2 (i.e. triple negative) and showed high proliferative index (Figure 4).




Table 1, Table 2 depict the clinicopathological features of the different molecular subtypes of BC. HER-2 enriched (66.7%; 6/9) and Basal like tumors (69.6%; 16/23) were relatively more frequent in ≤ 50 years of age in contrast to Luminal A (53.5%; 23/43) and B tumors (60%; 6/10). However, this association was not statistically significant (p= 0.614).
Invasive ductal carcinoma was the major histologic type across all the molecular subtypes. However, all the invasive lobular carcinomas and 83.3% of mucinous carcinomas were Luminal A and all the medullary carcinomas were Basal like.
Statistically significant association was found between molecular subtype and grade, with majority of grade 1 tumors (79.2%; 19/24) being Luminal A and majority of grade 3 tumors (58.8%; 10/17) being Basal like (p= 0.004).
Luminal A tumors (53.5%) exhibited less frequent lymph node metastasis, in contrast to HER-2 enriched (77.8%) and Basal like (82.6%) tumors. However, this association fell short of statistical significance (p= 0.139).
There was no statistically significant association between the molecular subtypes and pathological T stage and TNM stage (p= 0.855 for T stage and p= 0.78 for TNM stage).
Discussion
BC’s were originally classified into molecular subtypes based on gene expression profiling studies using DNA microarray-based applications. 8 However, a panel of IHC markers (including ER/PR, HER-2 and Ki-67) can be used as surrogate for gene expression profiling to classify tumors into molecular subtypes. 7, 8 In our study, based on immunophenotyping, we identified four molecular subtypes of BC.
The most prevalent molecular subtype in our study was Luminal A which is in synchrony with majority of the studies conducted in India and abroad (Table 3). 4, 5, 6, 9, 10, 11, 12, 13 However, in the study by Mittal et al. Luminal B was the commonest subtype. 14 The second commonest subtype in the current study was Basal like, which is also in synchrony with many other studies. 4, 5, 9, 10, 11, 12, 13 Among studies the frequencies of Luminal B ranged from 8.4% to 28% and HER-2 enriched ranged from 2.8% to 25% (Table 3). 4, 13, 14 In our study Luminal B and HER- 2 enriched subtypes were less frequent. Such minor geographical variations of molecular subtypes could be attributed to racial and environmental characteristics, genetic factors and technical and interpretative factors inherent in immunophenotyping. 5
|
Variables
|
Present study
|
Mittal et al. 14 |
Al- thoubaity et al. 5
|
Soni et al. 9 * |
Tiwari et al. 6 ** |
Widodo et al. 10 |
Four ati et al. 11
|
Cheng et al. 12 |
Kadivar et al. 4 |
Zaha et al. 13 |
|
Year of publication |
2022 |
2021 |
2020 |
2020 |
2015 |
2014 |
2014 |
2013 |
2012 |
2010 |
|
Place of study |
Karnataka, India |
Maharashtra, India |
Jeddah, Saudi Arabia |
Telangana, India |
Madhya Pradesh, India |
Yogyakarta, Indonesia |
Tunisia, North Africa |
China |
Tehran, Iran |
Timisoara, Romania |
|
Total no. of cases |
85 |
64 |
740 |
500 |
70 |
84 |
966 |
628 |
428 |
173 |
|
Durati on of study |
2 years |
3 years |
7 years |
2 years |
2 years |
- |
3 years |
- |
10 years |
5 years |
|
Luminal A |
50.60% |
22% |
58.50% |
38.20% |
27.10% |
38.10% |
50.70% |
46.50% |
63.8 |
53% |
|
Luminal B |
11.80% |
28% |
14% |
11.80% |
25.70% |
16.70% |
13.40% |
17.00% |
8.4 |
18.50% |
|
HER-2 enriched |
10.60% |
25% |
11.50% |
14.80% |
25.70% |
20.20% |
13.40% |
15.00% |
11.9 |
2.80% |
|
Basal like |
27% |
25% |
16% |
17% |
15.70% |
25% |
22.50% |
21.50% |
15.90% |
21.90% |
In studies conducted by Widodo et al. and Najfi et al. Luminal A and Basal like subtypes were respectively frequent in <50 and >50 years. 10, 15 Similarly we observed a greater preponderance of Basal like tumors (69.6%) in < 50 years of age in comparison to Luminal A tumors. However, this correlation was not statistically significant (p= 0.614). There was no significant association between age and molecular subtype in the series conducted by Soni et al. and Tiwari et al. 6, 9
Tumor size is an important prognostic factor and a predictor of relapse and dissemination in node-negative BC. 7 This is because, with increasing tumor growth there is higher lymphatic and vascular spread. 7, 13 Widodo et al. found that HER-2 enriched and Basal like tumors were significantly associated with large tumor size. 10 Al-thoubaity et al. and Tiwari et al. observed that Luminal A tumors were frequently small unlike HER-2 enriched, which were comparatively larger. 5, 6 However, similar to the studies conducted by Kadivar et al. and Soni et al., we didn’t find significant association between tumor size and molecular subtypes. 4, 9
Literature review reveals that lobular carcinomas are frequently associated with Luminal A subtype and some studies have found predominance of Medullary carcinomas with Basal like subtype. 4, 5, 6, 7, 9 In our study all the invasive lobular carcinomas and all the medullary carcinomas were respectively found to exhibit Lumina A and Basal like phenotype. However, these tumors were too infrequent, to test for statistical significance.
Histologic grade represents the morphological evaluation of biological features of BC and is an important prognostic factor. 16 Molecular profiling has revealed that grade reflects the molecular features better than the tumor size and lymph node status. 16 Many studies have found significant correlation between molecular subtype and grade, with Luminal A being frequently grade 1 (low grade/ well differentiated) and HER-2 enriched and Basal like being commonly grade 3 (high grade/ poorly differentiated). 4, 5, 6, 10, 14, 15 We observed a similar statistically significant association between molecular subtype and grade, with majority of grade 1 tumors being Luminal A and majority of grade 3 tumors being Basal like. In contrast, Setyawati et al. observed that all molecular subtypes were frequently of grade 3, probably attributed to the delayed diagnosis of BC in this study. 17
In synchrony with studies conducted by Kadivar et al., Soni et al., Mittal et al. and Setyawati et al., we did not find any significant association between molecular subtypes and lymph node metastasis. 4, 9, 14, 17 Al-thoubaity et al. reported a strong association between molecular subtype and lymph node metastasis, with HER-2 enriched cases showing a high frequency of lymph node metastasis. 5 Soni et al. and Walke et al. observed high frequency of nodal metastasis in HER-2 enriched and Basal like subtypes. 9, 18 Such discrepancy among studies, indicates that the molecular subtypes are intrinsic and thus loosely associated with nodal metastasis, as has been suggested by some studies. 5
We found that there was no significant association between TNM stage and molecular subtype. Tiwari et al. reported that Luminal A tumors are associated with early stage unlike the Luminal B, Her-2 enriched and Basal like subtypes, which are associated with higher stage. 6 Widodo et al. observed a statistically significant association between molecular subtype and stage. 10 These differing results among studies can be attributed to unequal distribution of cases across the stages. In order to determine the association between stage and molecular subtype, studies with equal number of cases in each stage have to be done. 10
One of the limitations of the study was the small number of cases in some of the histologic and molecular subtypes, which would have influenced the statistical analysis and could have affected the statistical power.
Conclusion
We have investigated the prevalence of the different molecular subtypes of BC and their association with certain demographic and prognostic factors. Luminal A tumors are the most prevalent molecular subtype and HER-2 enriched tumors, are the least prevalent. Correlation exists between histologic grade and molecular subtype with low-grade tumors frequently exhibiting Luminal A profile and high-grade tumors being frequently Basal like. There is no association of molecular subtype with age, tumor size, nodal metastasis, pathological T stage and TNM stage. Further, as literature review reveals variable correlation between the latter prognostic factors, larger and more representative cases with survival data should be evaluated. Finally, better perception of molecular subtypes of BC may pave way for targeted therapy and patient specific therapeutic approach.