Introduction
Burkholderia is an aerobic gram negative, non-fermenting bacteria that is widely dispersed in both natural and man-made habitats. The species ‘cepacia’ was first described by Walter H. Burkholder in mid-1940 as a cause of onion rot. There are 22 different species described under the genus Burkholderia. 1 Burkholderia cepacia is classified in 10 serovars that are collectively termed as Burkholderia cepacia complex (BCC). 2
The organism is known to cause infection in patients with cystic fibrosis, causes pneumonia, nosocomial infections noted as blood stream infections (BSI). BCC also causes infection in patients with various malignancies and those undergoing treatment for malignancies were also found to have BSI with BCC. 3, 4, 5
BCC is an opportunistic group, that is known to cause increased morbidity and mortality especially in hospitalised patients owing to its resistance to antibiotics. 6 The oragnism’s natural tendency to colonise various surfaces, intra-venous sets, central-lines, nebulizers, respiratory devices and disinfectants makes it a major cause for small hospital outbreaks. 7
Here, we present a series of five cases where an unusual violet pigment producing BCC was identified. To the best of our knowledge this is the first case of violet pigment producing BCC from Mumbai.
Case History
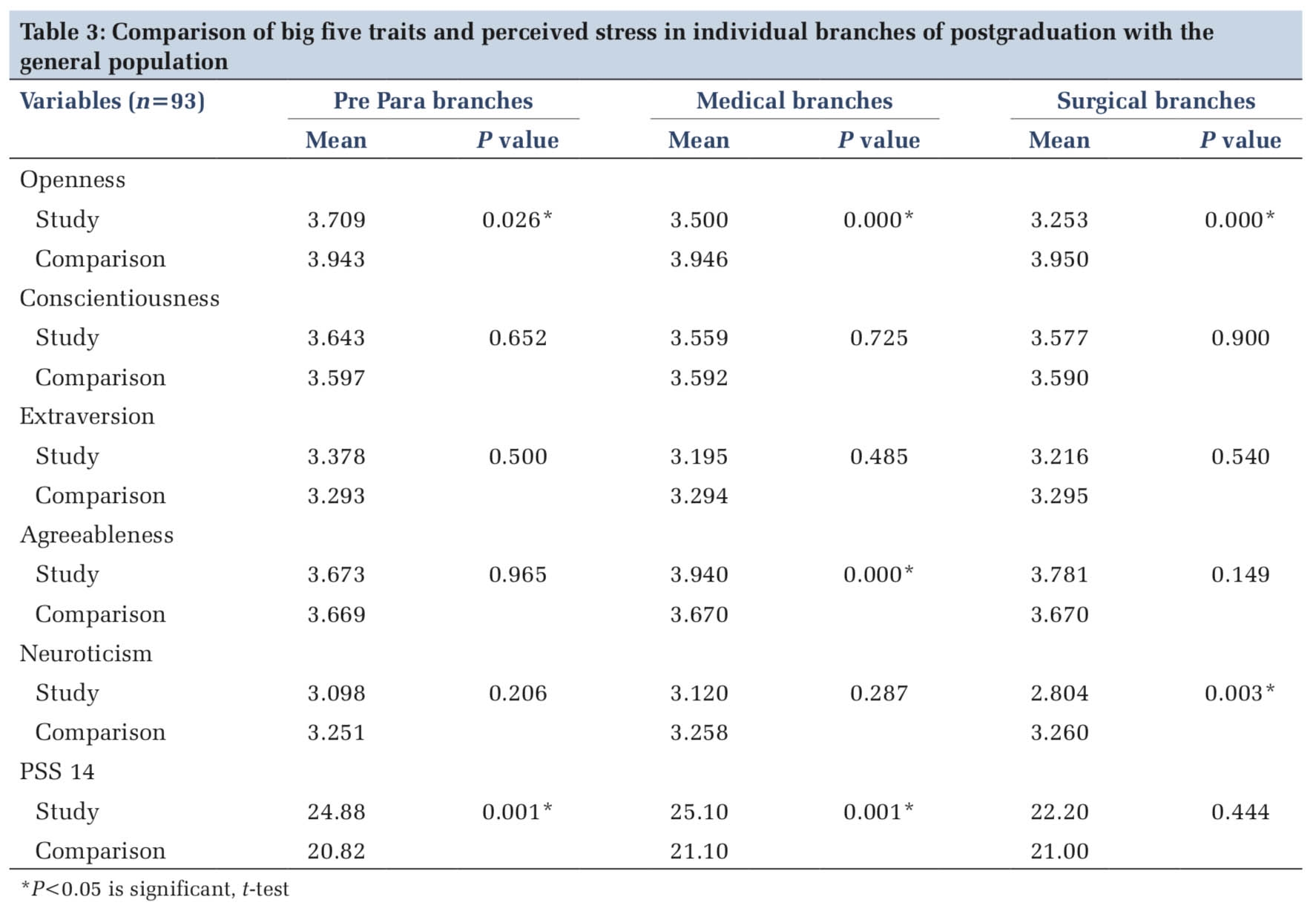
Relevant demographic characteristics and risk factors of five cases of sepsis were assessed and analyzed. This included age, sex, admitted in ward or intensive care unit (ICU), relevant clinical history, clinical co-morbidities, antibiotic treatment, exposure and duration of exposure to central venous access, history of indwelling devices and investigations done. These are depicted in Table 1.
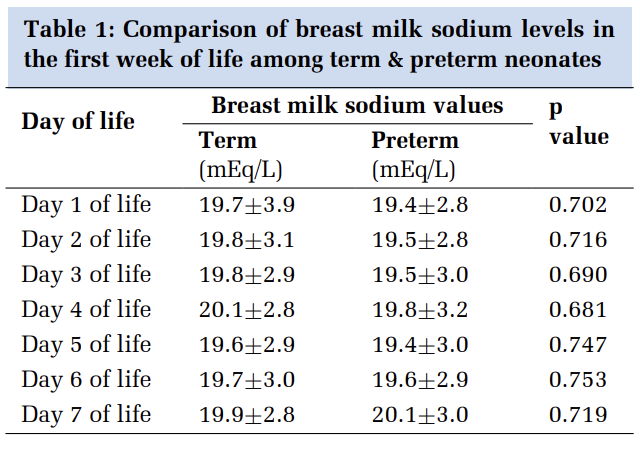
In all the above mentioned five cases, laboratory diagnosis of sepsis was confirmed by blood culture. Blood samples were processed in BACTEC 9120 system. Subcultures were done on Blood agar and MacConkey agar plates from flash positive bottles and the plates were incubated overnight at 370C. 8 Phenotypic identification was done with Vitek 2 ID-GNB card (BioMérieux, India) from a private laboratory. Antimicrobial susceptibility test (AST) was performed by Kirby Bauer Disc Diffusion Method (KBDDM) on Mueller Hinton Agar (MHA), according to CLSI guidelines 2018. 9 Antibiotic discs used were Meropenem (10µg), Cotrimoxazole (1.25/23.75µg), Ceftazidime (30µg), Gentamicin (30µg) and Polymyxin B (300U).
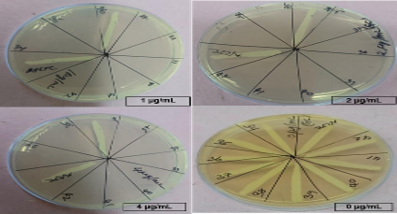
Culture on MacConkey Agar demonstrated growth of unusual violet colored pigmented colonies (Figure 1 a). The colonies were 0.5 - 1 mm in size, non-lactose fermenting, circular with entire edge and regular margins, in contrast to typical colonies of BCC. (Figure 1 b & c) show the violet coloured pigment on Nutrient Agar & Blood Agar respectively. All the five isolates obtained had a similar morphological appearance and growth characteristics. Burkholderia cepacia complex was identified in blood cultures of all the five patients by Vitek 2 identification systems. All the isolates were sensitive to meropenem, cotrimoxazole and ceftazidime but were resistant to gentamicin and polymyxin B (Figure 1d).
|
Sr No. |
Age/ Sex |
ICU/ Ward |
Relevant clinical history |
Indwelling device (CVA or Catheter) |
Other investigations |
Diagnosis |
Antibioitcs Before (Antibiotic susceptibility report) ABS report |
Antibiotics after ABS report |
|
1. |
57/M |
ICU |
-History of highgrade fever post chemotherapy -History of platelet and blood cell transfusion -No history of diabetes or hypertension |
Put on Mechanical ventilator for 2 days |
Flow Cytometry: Phenotypes- CD13, CD14, CD 11b, CD33,CD64, CD36, CD38, CD34, CD45, CD117, HLA-DR. 2D ECHO: Degenerative affection of aortic valve, Mild pulmonary hypertension USG: Hepatomegaly with diffuse fatty liver Peripheral blood smear: Blastocyte 6%, Promyelocyte 30%, Myelocyte 8%, Metamyelocyte 4%. |
Acute Myeloid leukemia (AML), AML-M4 |
On chemotherapy plus Imipenem, Amphotericin B, Colistin |
Started on Ceftazidime, Cotrimoxazole and Continued with Inj Imipenem |
|
2. |
50/M |
MICU |
-History of cough, expectoration, Breathlessness, hemoptysis and high grade fever -No history of diabetes or hypertension |
- |
Sputum for AFB: Negative 2D ECHO: Mitral valve prolapsed with mitral regurgitation with moderate pulmonary hypertension, LVEF- 25% HRCT: Patchy areas of consolidation in right lower lobe. |
Congestive Cardiac failure with mitral regurgitation with lower respiratory infection |
Inj piperacillin-tazobactam Inj frusemide, Inj levofloxacin Tab. Digoxin Tab. Carvedilol |
Inj Meropenem Inj Cotrimoxazole Inj Ceftazidime, in addition to ongoing antibiotics |
|
3. |
53/F |
Medicine Ward |
-Chest pain and fever with chills -No history of diabetes mellitus and hypertension |
- |
2D ECHO: Atrial fibrillation, Tricuspid regurgitation, LVEF- 30% |
Rheumatic heart disease |
Tab. Prolomet Tab. Warfarin |
Inj Meropenem Inj Cotrimoxazole Inj Ceftazidime, in addition to ongoing treatment |
|
4. |
55/M |
MICU |
-History of breathlessness, nausea, blood in vomitus and stool -No history of diabetes mellitus and hypertension |
- |
2D ECHO: mitral stenosis ,mild mitral valve regurgitation and prolapse, LVEF- 30% |
Rhuematic Heart Disease with Warfarin Toxicity With Upper GI Bleed |
Tab. Diazepam Inj vitamin K, Inj Lasix Tab. Prolomet |
Inj Meropenem, in addition to ongoing treatment |
|
5. |
47/F |
MICU |
History of breathlessness , pain in abdomen , pedal edema Known case of diabetes mellitus -No history of hypertension |
- |
USG abdomen & pelvis: suggestive of renal infract and mesentric vessel thrombosis. |
Known case of Rheumatic Heart Disease |
Inj ceftriaxone, inj dopamine, inj digoxin, inj heparin, |
No change in treatment |

Discussion
BCC are gram negative bacteria belonging to β proteobacterium subdivision. They are notorious opportunistic pulmonary pathogen commonly infecting patients suffering from chronic granulomatous diseases and cystic fibrosis. Due to its ability to thrive in the diverse range of environments, BCC contributes to increased morbidity and mortality in hospitalized patients. Various outbreaks of BCC septicemia are seen in hospitalized patients – both immunocompetent and immunosuppressed, as well in ICUs and oncology units. 10
Patients with nosocomial infections, cystic fibrosis, those with indwelling catheters and on ventilators should all raise a suspicion for BCC bacteremia. Patients with cystic fibrosis and having colonisation with BCC have progressive, fatal and reduced survival time, leading to ‘cepacia syndrome’ in many cases as compared with non-colonised BCC. In non-cystic fibrosis patients, BCC causes pneumonia, urinary tract infections, bloodstream infections and meningitis, endocarditis and malignant conditions like AML. 11, 12
Owing to the wide distribution and survival in different environment, this pathogen has a tendency to cause nosocomial infections and hospital outbreaks. Studies show that BCC isolation has been found from iv solutions, nebulisation solutions, contaminated medicines etc. 13
Burkholderia cepacia complex is intrinsically resistant to polymyxin and also show increasing multi-drug resistance, 14 which makes it a notorious pathogen to treat in critically ill and ICU patients.
In all the five cases, the isolated colonies showed violet pigment production which was a unique feature shown by members of Burkholderia cepacia complex. This is supported in a study by Rastogi et al 15 and Ranjan et al 16 which also highlights the violet pigment producing BCC from blood cultures.
Extensive study and literature on the phenotypic and genotype of various Burkholderia species need to be studied to know the cause of such diversity in its appearance.
This is probably the first study from Mumbai showing violet color pigment producing Burkholderia cepacia complex.
Conclusion
Burkholderia cepacia complex are most commonly found in association with hospital acquired infections. However we have come across five cases of sepsis caused by this pathogen producing an unusual violet colored pigment.