

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2019.v05i02.001
Year: 2019, Volume: 5, Issue: 2, Pages: 1-8
Original Article
Elias Adikwu, Nelson Clemente Ebinyo, Harold Aagbadabina
Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria
Address for correspondence:
Dr. Elias Adikwu, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria. E-mail: [email protected]
Aim: The efficacy and therapeutic benefit of flutamide (FLU) can be clinically limited by the occurrence of hepatotoxicity which may progress to liver failure. This study, therefore, evaluated the protective effect of coenzyme Q 10 (COQ 10) against FLU-induced liver toxicity in albino rats.
Objectives: The objectives of this study were to explore and grade the severity of IA in relation to academic performance among medical undergraduates.
Materials and Methods: Forty-five adult albino rats of average weight 200–250 g were randomized into groups and treated intraperitoneally with COQ 10; 50 mg/kg + FLU 200 mg/kg, COQ 10; 100 mg/kg + FLU 200 mg/kg and COQ 10; and 200 mg/kg + FLU 200 daily for 7 days. On the 8th day, rats were sacrificed, serum was extracted from blood and liver was excised and evaluated for aspartate aminotransferase (AST), alanine aminotransferase(ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), lactate dehydrogenase (LDH), total bilirubin (TB), and conjugated bilirubin (CB). Liver was evaluated for oxidative stress markers and pathologic changes.
Results: Serum and liver AST, ALT, ALP, AST, GGT, LDH, TB, and CB levels were significantly (P < 0.001) increased in FLU-treated rats when compared to placebo control. Liver superoxide dismutase, catalase, glutathione, and glutathione peroxidase were decreased, whereas malondialdehyde levels were increased significantly (P < 0.001) in FLU-treated rats when compared to placebo control. The liver of FLU-treated rats showed hepatocyte necrosis. However, FLU-induced liver toxicity was significantly abrogated in a dose-dependent manner in COQ 10; 50 mg/kg (P < 0.05), COQ 10; 100 mg/kg (P < 0.01); and COQ 10; 200 mg/kg (P < 0.001) pre-treated rats when compared to FLU-treated rats.
Conclusions: This study showed that COQ 10 abrogates FLU-induced hepatotoxicity in albino rats in a dose- dependent manner. It may have clinical application in hepatotoxicity associated with FLU.
KEY WORDS:Antioxidant, flutamide, liver, protection, rat, toxicity.
Flutamide (FLU) which competitively antagonizes androgen receptor is used in combination with castration in the treatment of prostatic cancer. It competes for the androgen receptor in the prostate gland with testosterone hormone and its biotransformed and active metabolite; dihydrotestosterone. Its action can inhibit the stimulatory effects of testosterone hormone and its active metabolite dihydrotestosterone on prostate cancer cells, thereby preventing the growth and spread of prostate cancer cells.[1] However, the efficacy and therapeutic benefit of FLU can be clinically limited by the occurrence of hepatotoxicity in prostate cancer patients. Hepatotoxicity due to therapy with FLU includes acute hepatitis, which may progress to a fulminant form (acute liver failure) that may require liver transplantation. It is often characterized by elevations in liver enzymes and in some cases alterations in liver histology. The incidence of hepatotoxicity associated with FLU has been reported to be 6–9% in prostate cancer patients.[2] This instigated Food and Drug Administration to ask for a black box warning on FLU label to highlight the possible development of hepatotoxicity during therapy. The precise mechanism by which FLU causes hepatotoxicity is poorly understood; however, some studies attributed it to FLU metabolites which can initiate and propagate free radical production leading to hepatic oxidative stress (OS) and consequently to lipid peroxidation (LPO) and hepatic injury.[3] Furthermore, in vitro and in vivo studies have associated FLU with the generation of free radicals in liver tissues.[4] Furthermore, some studies suggested the involvement of mitochondrial dysfunction as indicated by altered mitochondrial morphology, mitochondrial membrane potential loss, and ATP depletion in FLU-induced hepatotoxicity.[5]
Coenzyme Q10 (COQ10) is a biochemical compound that is endogenously produced and is present in human cells. It has a benzoquinone ring and a polyisoprenoid tail that contains 6 to 10 subunits which are species-specific and enhances its stability.[6] It is a redox-active lipophilic free radical scavenger that is present in cellular organelles such as lysosomes, mitochondria, and Golgi vesicles.[7] In mitochondria, COQ1O plays an important role in the production of energy by functioning as an electron carrier in the electron transport chain.[8] Outside the mitochondria, it functions as an essential antioxidant by scavenging free radicals and working in synergy with other antioxidants. It can abrogate the initiation and propagation of free radicals, thereby inhibiting the detrimental effects of free radicals on lipids, proteins, DNA, and the functionality of mitochondria.[9,10] Furthermore, in vitro studies using animal models have reported the activities of COQ10 as an anti-inflammatory agent. Other activities of COQ 10 include modulation of the functions of the immune system[11] stabilization of membrane and modulation of gene expression.[12] COQ10 has gained attention due to its possible therapeutic effects on certain diseases such as neurodegenerative diseases, cancer, cardiovascular disease, and diabetes.[13] The current study, therefore, assessed if pretreatment with COQ10 can protect against FLU-induced hepatotoxicity, which has not been evaluated.
Forty-five adult albino rats, weighing 200–250g were sourced from the animal house of the Faculty of Pharmacy, University of Port Harcourt, Nigeria. The rats were domiciled in the animal house of the Faculty of Pharmacy, Niger Delta University, Nigeria, where the study was performed. The rats were housed at a temperature of 23 ± 2°C with a cycle of 12 light/12 dark and were allowed to acclimatize for 2 weeks before the experiment with free access to standard diet and water ad libitum. This study was approved by the Ethics Committee of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria. The rats were handled according to the guidelines of the United States National Academy of Sciences Guide for the Care and Use of Laboratory Animals.
Dose selection and animal treatment
COQ (50 mg/kg, 100 mg/kg, and 200 mg/kg) dissolved in corn oil[14] and FLU (200 mg/kg)[15] were used for this study. The rats were assigned randomly to nine groups (A-I) of n = 5. Groups A and B served as placebo and solvent control and were treated intraperitoneally (i.p) with water and corn oil, for 7 days respectively, whereas groups C-E were treated i.p with COQ 10 (50 mg/kg, 100 mg/kg, and 200 mg/kg) daily for 7 days respectively. Group F was treated i.p with FLU 200 mg/kg daily for 7 days. On the other hand, groups G-1 were treated i.p with COQ 10; 50 mg/kg + FLU 200 mg/kg, COQ 10; 100 mg/kg + FLU 200 mg/kg and COQ 10; 200 mg/kg + FLU 200 mg/kg daily for 7 days respectively.
Animal sacrifice
On the 8th day of the study, rats were euthanized with diethyl ether, and blood sample was drained from the heart. The blood was collected in a plain sample container and allowed to clot at room temperature. The serum was extracted from the clot by centrifugation at 3000 rpm for 15 min and used for the evaluation of biochemical parameters. Liver was harvested rinsed in ice cold saline. It was sliced, minced and homogenized in 10 mM Tris-HCl buffer, pH 7.4 (10%w/v) with the aid of Teflon pestle homogenizer at a speed of 2500 rpm. The clear supernatant was collected and used for the assessment of OS markers and aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and lactate dehydrogenase (LDH).
Evaluation of liver function markers
Evaluations of serum and liver AST, ALT, ALP, GGT, LDH, total bilirubin (TB), and conjugated bilirubin (CB) were carried out using standard laboratory test kits according to manufacturer’s specification.
OS marker assay
Liver malondialdehyde (MDA) was assayed, according to Buege and Aust[16] whereas reduced glutathione (GSH) was assayed as reported by Sedlak and Lindsay[17] Superoxide dismutase (SOD) was determined as reported by Sun and Zigma[18] whereas catalase (CAT) was evaluated according to Sinha et al., 1972.[19] GSH peroxidase (GPX) was determined according to Rotruck et al., 1973.[20]
Histological examination of the liver
Rats were dissected after euthanasia; liver was harvested, blotted free of blood, and fixed in 10% formalin solution for 24 h. The fixed liver tissues were dehydrated with the aid of ascending alcohol solutions then cleared in xylene. Tissues were embedded in paraffin blocks and sectioned with the aid of a microtome. 5 μm thick sections of the tissues were stained with hematoxylin and eosin. Stained sections were examined with the aid of a light microscope, and relevant sections were photographed.
Statistical analysis
Results were statistically evaluated by two-way ANOVA using SPSS 20.00, followed by Tukey’s post hoc test. Results are expressed as the mean ± standard error of mean. P < 0.05, 0.01, and 0.001 were considered significant.
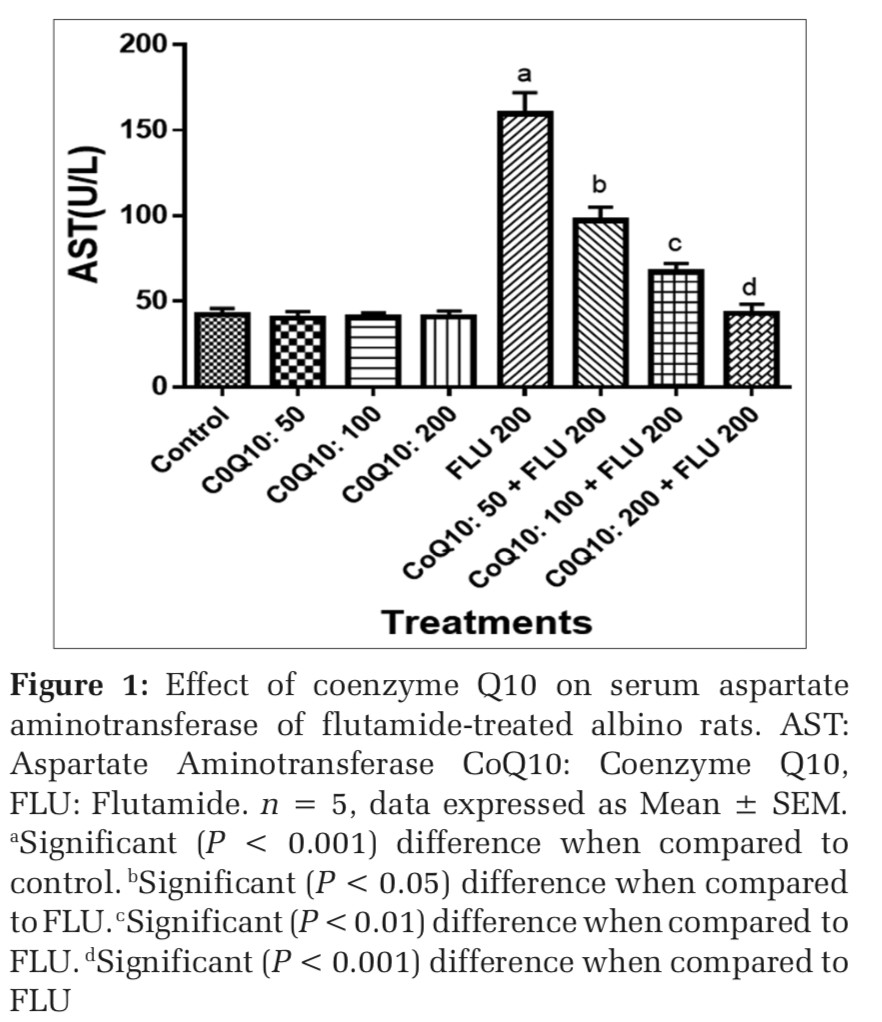
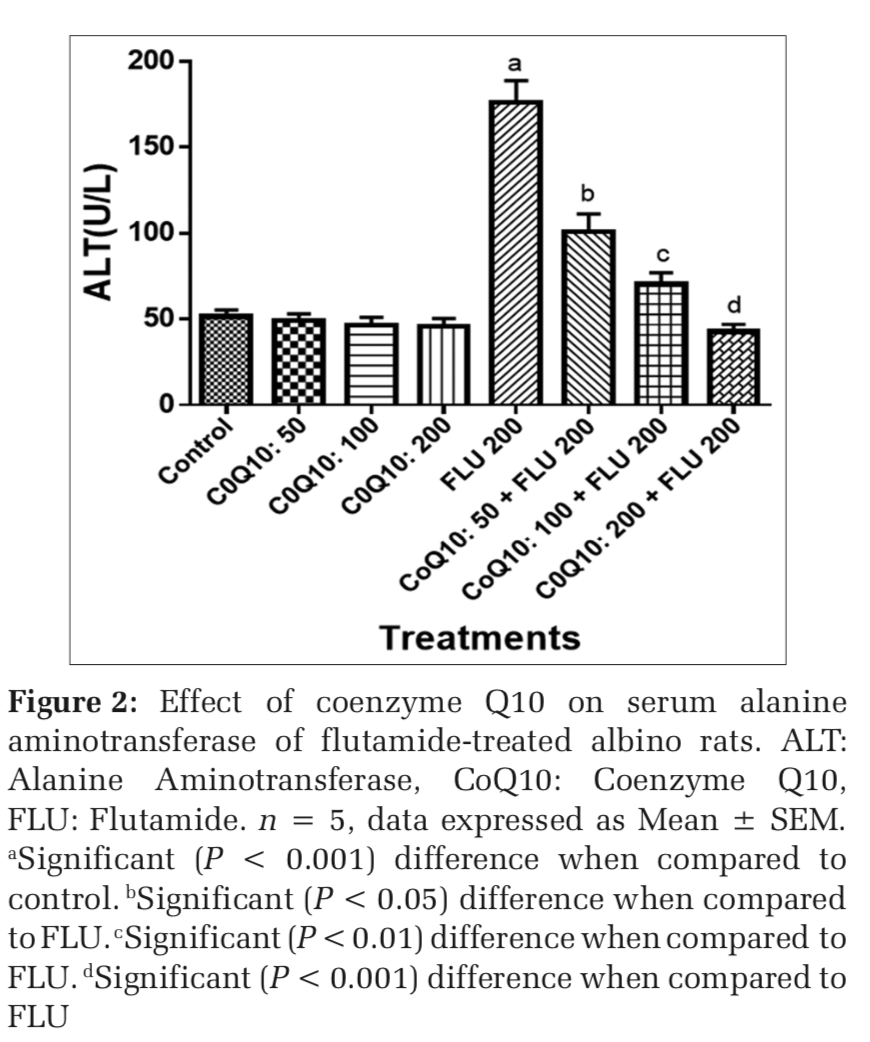
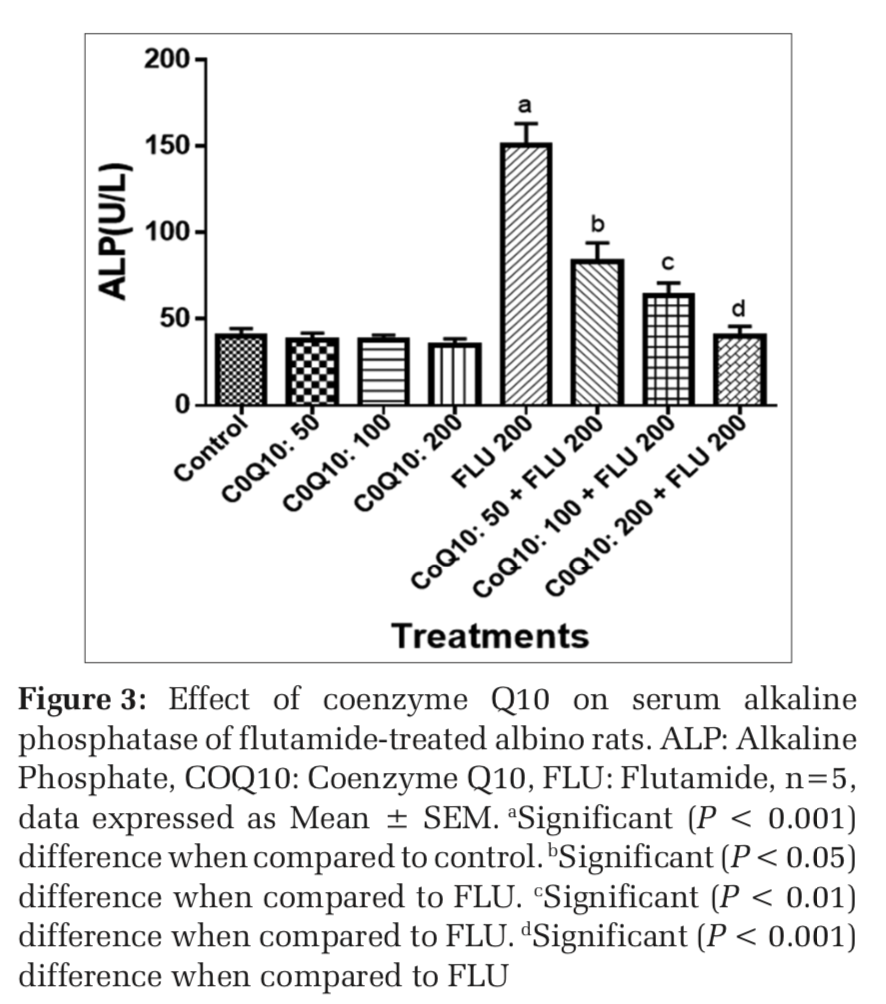
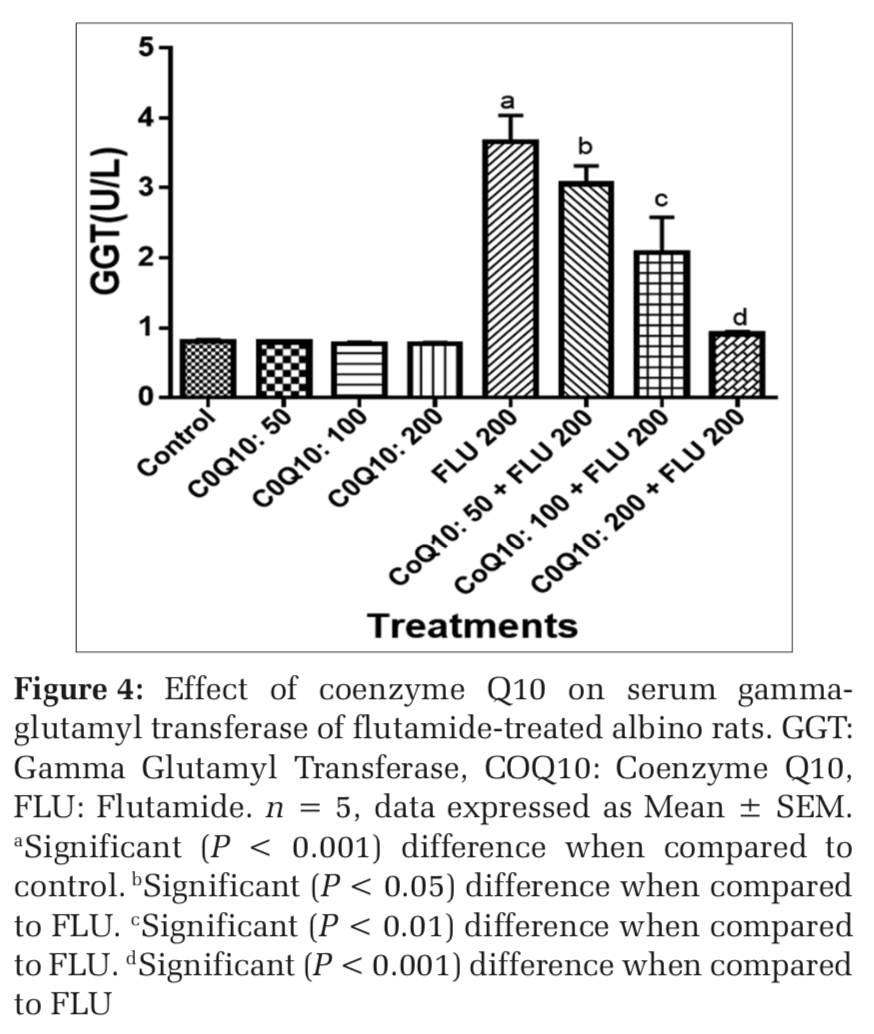
Effects on COQ 10 on liver function markers of FLU-treated rats
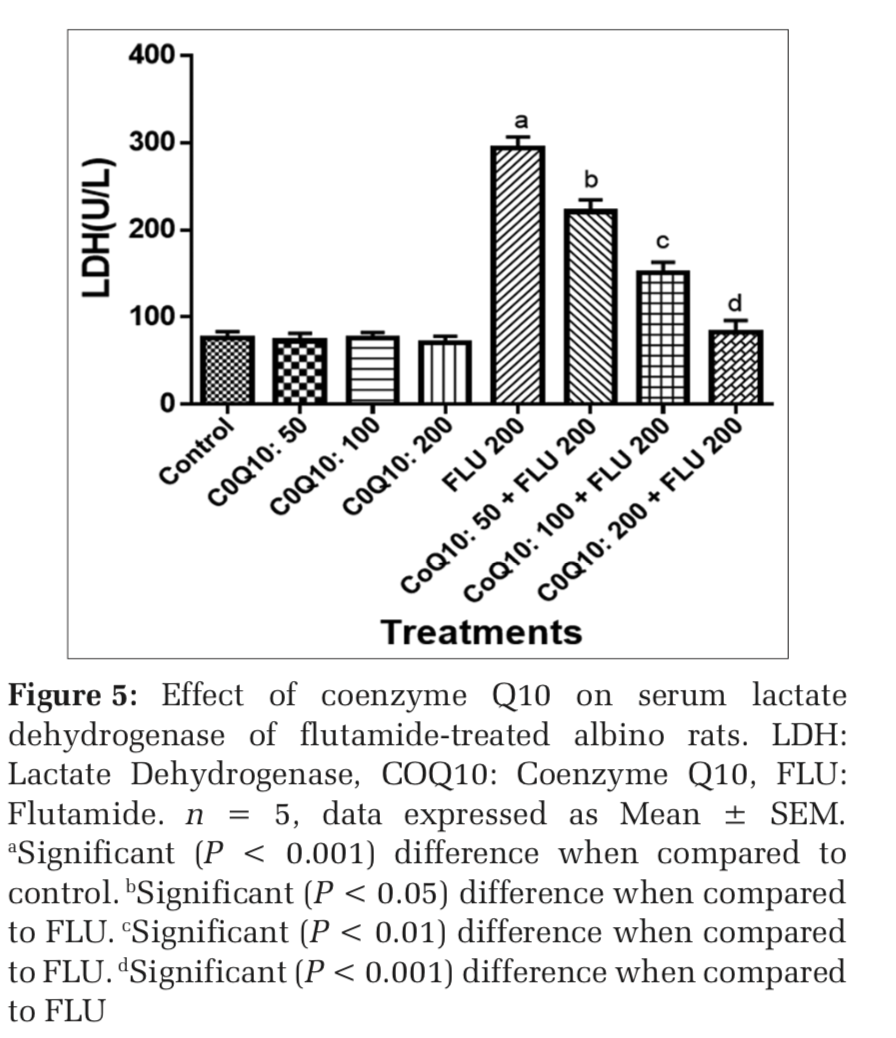
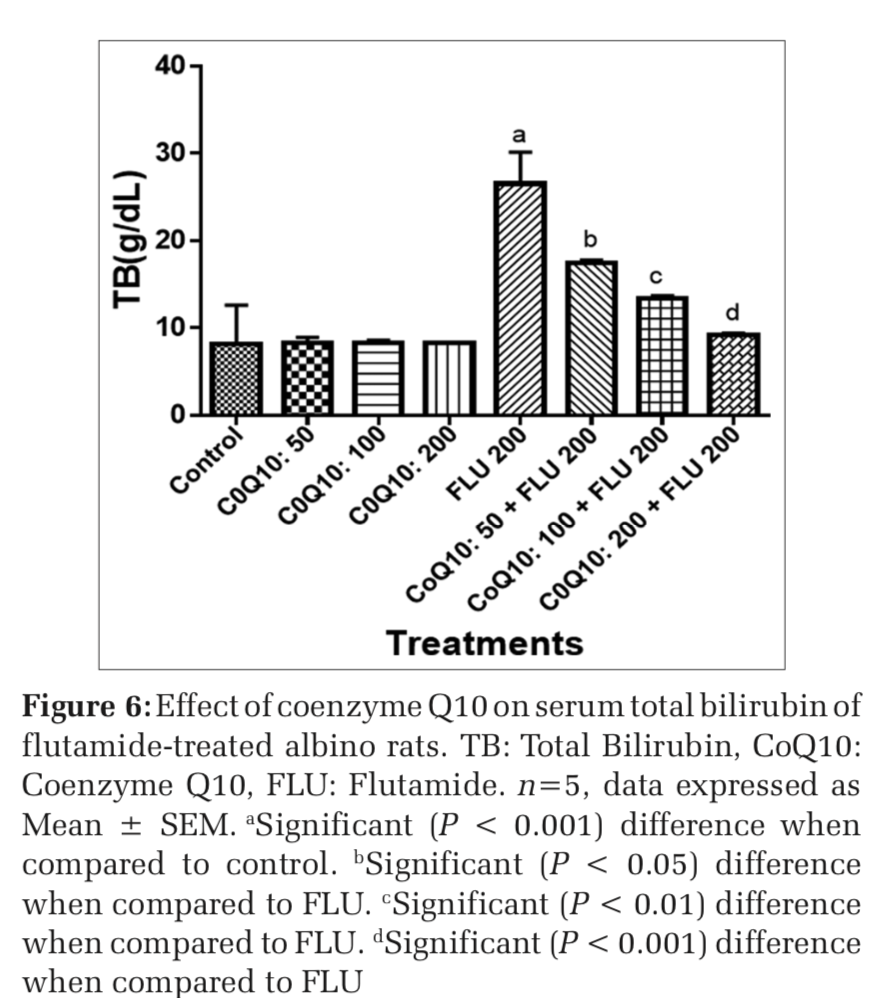
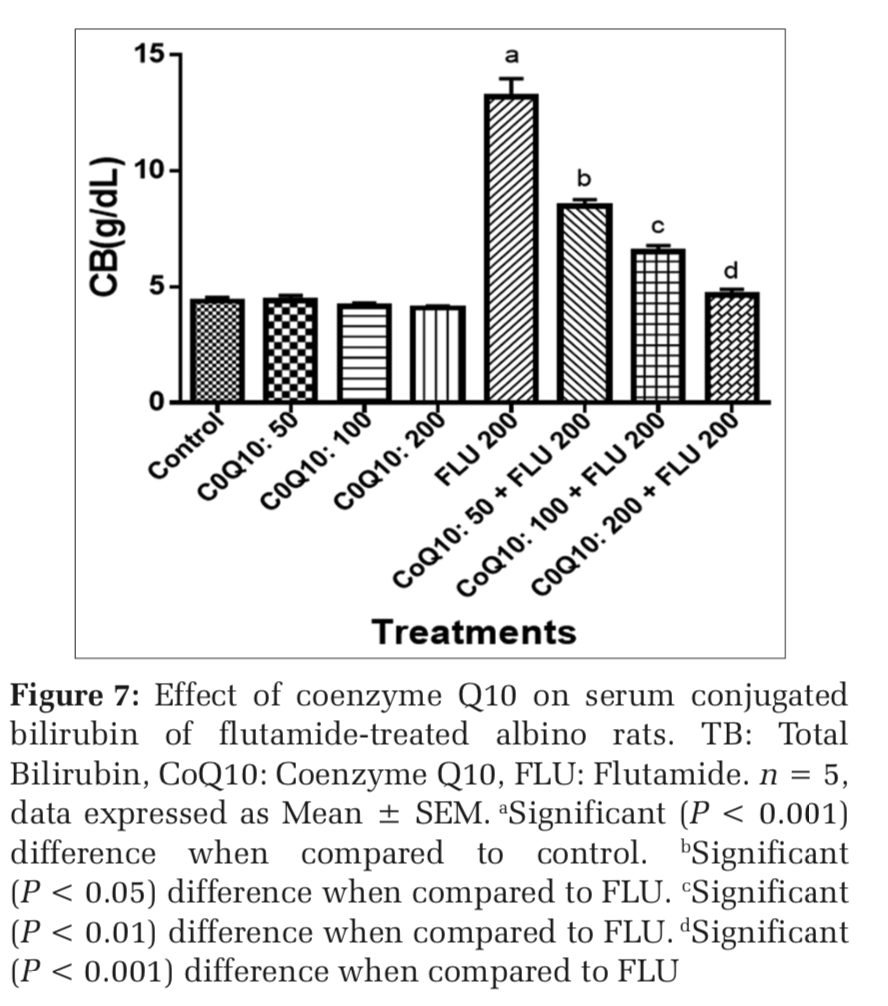
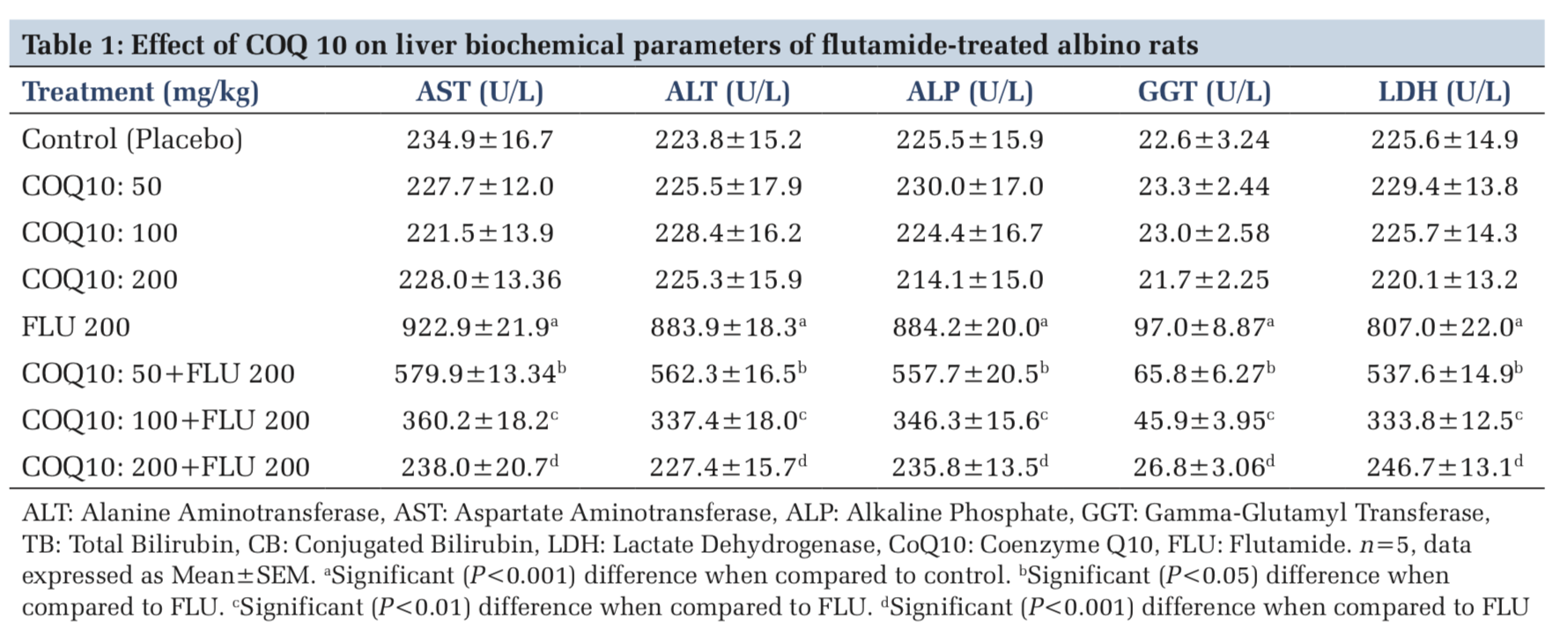
The administration of COQ 10 (50 mg/kg, 100 mg/kg, and 200 mg/kg) did not produce significant (P > 0.05) effects on serum AST, ALT, ALP, GGT, LDH, TB, and CB when compared to placebo control (Figures 1-7). However, the aforementioned parameters were significantly (P < 0.001) elevated in rats treated with FLU 200 mg/kg when compared to control. Interestingly, the serum levels of AST, ALT, ALP, GGT, LDH, TB, and CB were decreased in a dose-dependent fashion in rats treated with COQ 10; 50 mg/kg + FLU 200 mg/kg (P < 0.05), COQ 10; 100 mg/kg + FLU 200 mg/kg (P < 0.01), and COQ 10; 200 mg/kg + FLU 200 mg/kg (P < 0.001) when compared to rats treated with FLU 200 mg/kg (Figures 1-7). Normal (P>0.05) levels of AST, ALT, ALP, GGT, and LDH were obtained in the liver of COQ 10 (50, 100, and 200 mg/kg) treated rats when compared to control (Table 1). However, the above parameters were significantly (P < 0.001) increased in rats treated with FLU 200mg/kg when compared to control (Table 1). It is noteworthy that AST, ALT, ALP, GGT, and LDH levels were significantly decreased in a dose-dependent manner in COQ 10; 50 mg/kg + FLU 20 mg/kg (P < 0.05), COQ 10; 100 mg/kg + FLU 200 mg/kg (P < 0.01), and COQ 10; 200 mg/kg + FLU 200 mg/kg (P < 0.001) treated rats when compared to rats treated with FLU 200 mg/kg (Table 1).
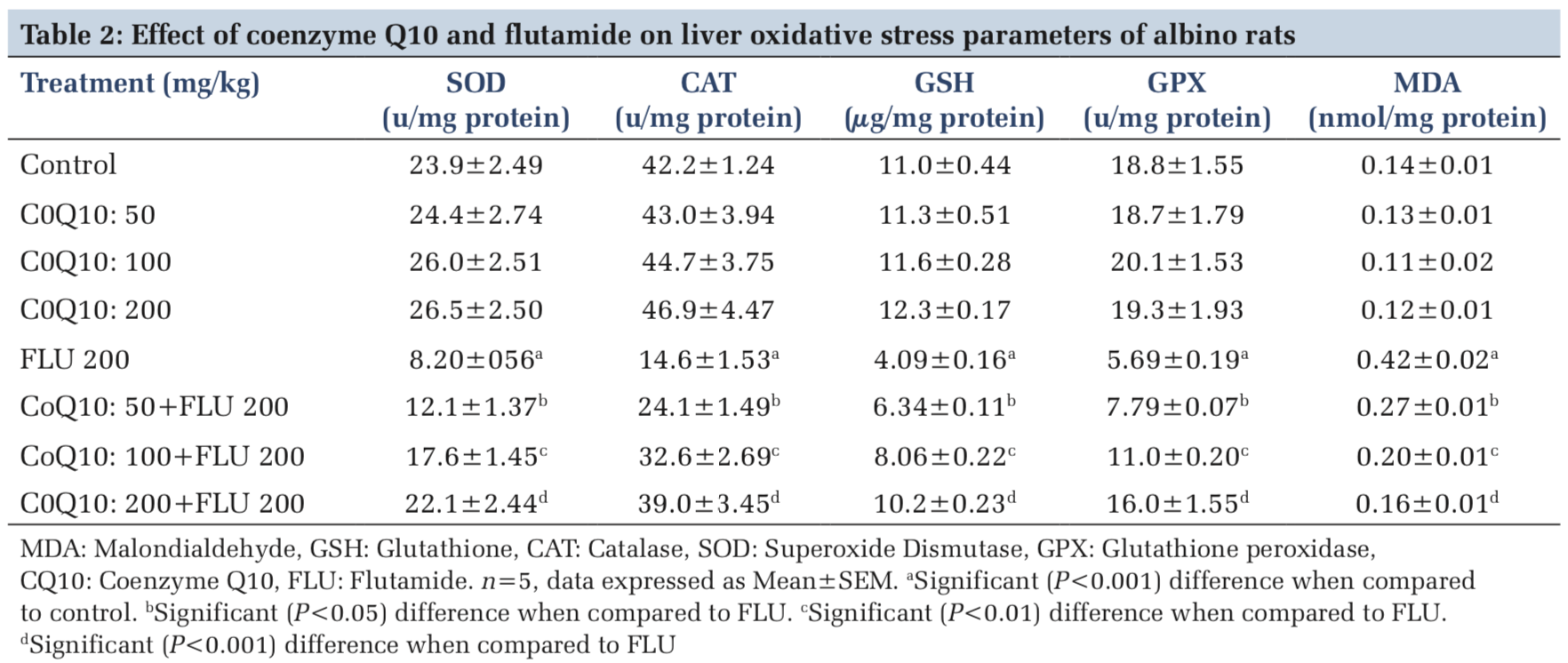
Effects of COQ 10 on liver OS indices and histology of FLU-treated rats
Furthermore, the liver levels of SOD, CAT, GSH, GPX, and MDA were normal (P > 0.05), in rats administered with COQ 10 (50 mg/kg, 100 mg/kg, and 200 mg/kg) when compared to placebo control (Table 2). On the other hand, the liver levels of SOD, CAT, GSH, and GPX were significantly (P < 0.001) decreased whereas MDA levels were significantly (P < 0.001) increased in rats treated with FLU 200 mg/kg when compared to control (Table 2). However, the liver levels of SOD, CAT, GSH, and GPX were significantly increased, whereas MDA levels were significantly decreased in a dose-dependent manner in rats treated with COQ 10; 50 mg/kg + FLU 200 mg/kg (P < 0.05), COQ 10; 100 mg/kg + FLU 200 mg/kg (P < 0.01), and COQ 10; 200 mg/kg + FLU 200 mg/kg (P < 0.001) when compared to FLU 200 mg/kg treated rats (Table 2).
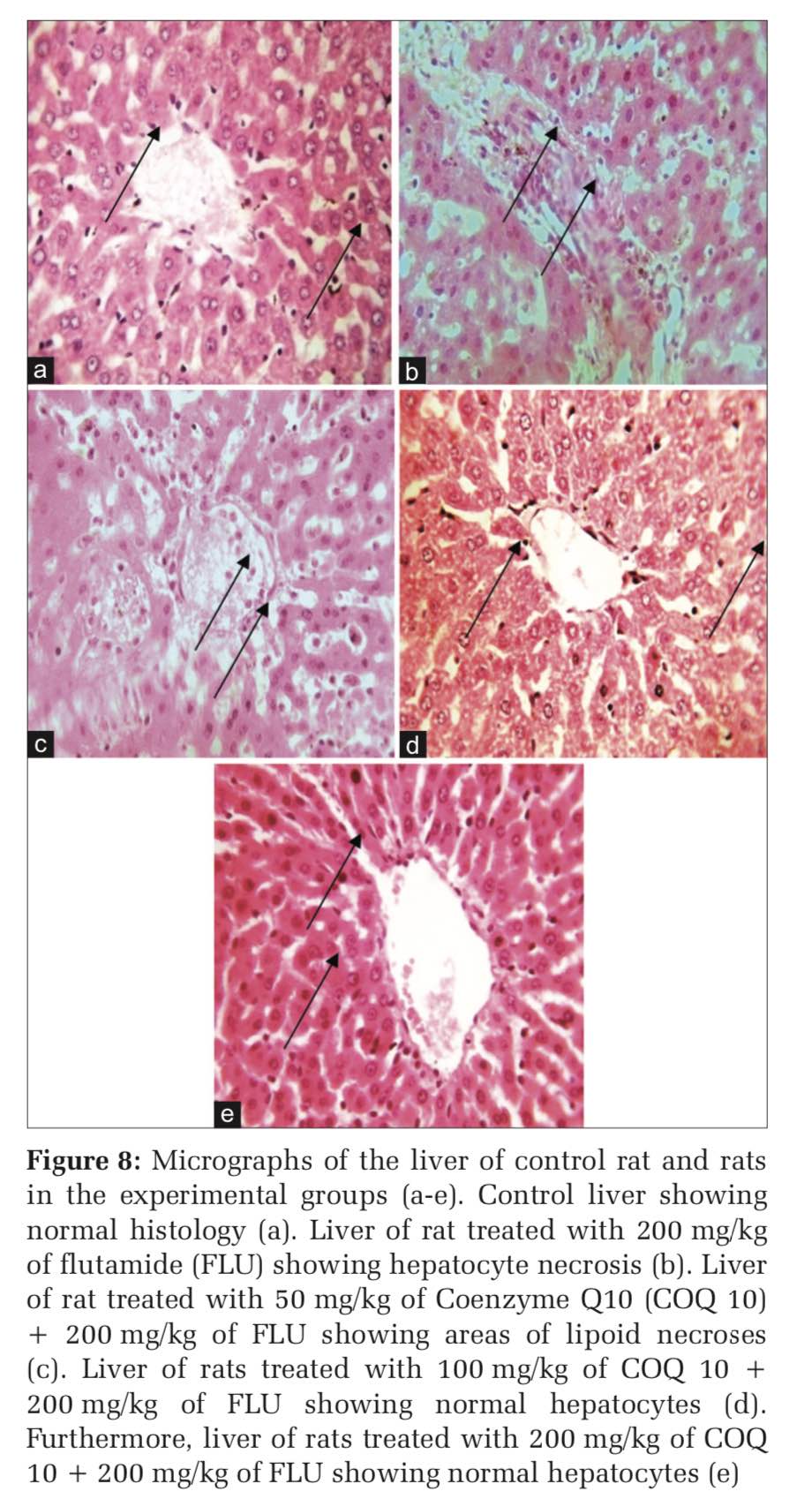
The control rat showed normal hepatocytes whereas rats treated with FLU 200 mg/kg showed hepatocyte necrosis (Figure 8a and b). On the other hand, rats treated with COQ 10; 50 mg/kg + FLU 200 mg/kg showed areas of lipoid necroses (Figure 8c). However, rats treated with COQ 10; 100mg/kg + FLU 200 mg/kg (Figure 8d) and COQ 10; and 200 mg/kg + FLU 200 mg/kg showed normal liver histology (Figure 8e).
The surge in the clinical use of FLU in the fight against prostate cancer is quite overwhelming with positive therapeutic outcome. The pains and daunting challenges that have bedeviled the scourge of prostate cancer have been nib in the bud by FLU. However, the hepatotoxic consequence that characterizes the clinical application of FLU could instigate a rethink and reassessment of risk/benefit ration in some quarters.[21] Furthermore, the limited or the relatively available treatment options for hepatic perturbation caused by FLU is a daunting challenge. The present study, therefore, deem it fit to open up a new frontier by evaluating if COQ10 can safeguard the liver of FLU intoxicated rats. In both clinical and experimental settings, ALT, ALP, AST, GGT, LDH, and bilirubin are among the determinants employed to ascertain the nature and extent of hepatic perturbations caused by xenobiotics. ALT is highly precise in monitoring hepatocellular status, whereas AST is a basic and sensitive yardstick for hepatic mitochondrial perturbation, especially around the centrilobular areas of the liver. In a scenario of compromised hepatic integrity, the aforementioned hepatic function markers are often elevated above the established clinical benchmark.[22] The current study observed functional compromise in the liver of FLU- treated rats as evidenced by elevated serum and liver levels of ALT, ALP, AST, GGT, LDH, CB, and TB. This observation is consistent with reported altered serum hepatic function markers due to treatment with FLU.[23] However, FLU-induced perturbation in hepatic function was abrogated in a dose-dependent fashion by pretreatment with COQ 10 as evidenced by the restored serum and liver levels of the aforementioned parameters.
OS through free radical generation is often regulated by the activities of SOD, CAT, GSH, GPX and other antioxidants thereby limiting the potential toxicities that may arise from their actions.[24] SOD catalyzes the dismutation of superoxide anion into molecular oxygen and hydrogen peroxide. Furthermore, it prevents peroxynitrite formation, mitochondrial, and endothelial dysfunction that may be associated with nitric oxide.[25] CAT scavenges free radicals and also protects cells against damage caused by lipoperoxides or hydroperoxides[26] whereas GSH directly or indirectly scavenges free radicals.[27] However, excess or uncontrollable activities of free radicals can overwhelm the opposing functions of SOD, CAT, GSH, and GPX, thereby culminating in OS and tissue damage. The current study observed hepatic OS marked by alterations in antioxidant status in FLU-intoxicated rats as evidenced by decreases in hepatic SOD, CAT, GSH, and GPX content. This finding is consistent with decreases in hepatic antioxidant status reported in FLU- intoxicated rats.[28] In contrast, hepatic antioxidant levels were restored in a dose-dependent manner in rats pretreated with COQ 10 characterized by vivid increases in SOD, CAT, GSH, and GPX levels.
LPO which plays an important role in pathological processes is a catalytic free radical-induced oxidative deterioration of polyunsaturated fatty acids in cells leading to lipid hydroperoxides formation and subsequently ketones, aldehydes, alkanals, alkenals, and MDA formations.[29] The extent and nature of injury caused by LPO can be measured by conjugated dienes, MDA, 4-hydroxynonenal, and others byproducts. However, MDA has been primarily used to evaluate and validate the occurrence of LPO.[30] The current study observed marked hepatic LPO characterized by higher MDA levels in FLU-treated rats. This observation is consistent with the reported increase in MDA level due to FLU intoxication.[31] Interestingly, the induction of LPO by FLU was abrogated in COQ10 supplemented rats in a dose-dependent manner.
Histopathology is the microscopic study of diseased tissues. It is an important investigative medical tool that is based on the study of human or animal histology with the aid of a microscope. It allows visualization of tissue and cell microscopic features and recognizes specific microscopic structural changes caused by diseases. It basically compares diseased or experimentally altered tissues with matching sample from healthy or control counterparts.[32] The histological assessment of the liver of rats administered with FLU showed altered liver morphology accompanied by hepatocyte necrosis. The observation is an agreement with altered hepatic morphology reported in previous studies.[33] It is quite fascinating that FLU-induced hepatocyte necrosis was abrogated in rats supplemented with COQ10 in a dose-dependent manner.
The mechanism by which FLU causes hepatotoxicity remains unclear; however, studies have associated it with its hepatic biotransformed product 2-hydroxy FLU (OH-FLU). In vitro studies have confirmed the toxic effect of OH-FLU on cultured rat hepatocytes. Furthermore, 5-amino-2-nitrobenzotrifluoride (FLU-1) a metabolite obtained from FLU hydroxylation may play a significant role in FLU-induced hepatotoxicity. Studies further suggested that FLU-induced hepatotoxicity can be associated with hydrolysis operated by an arylacetamide deacetylase on FLU.[34] Furthermore, the net effects of the aforementioned metabolites can be OS, inflammation, and mitochondria dysfunction.[35,36] In the current study, the ability of COQ 10 to safeguard the liver from FLU-intoxication can be attributed to its antioxidant and anti-inflammatory effects. Due to the antioxidant effect of COQ10, it might have scavenged free radicals and protected membrane phospholipids, mitochondrial membrane protein from OS and LPO-induced damage.[37] Furthermore, due to its anti-inflammatory effect, it might have inhibited the production of pro-inflammatory cytokines such as tumor necrosis factor-α and Interleukin-1β that are associated with hepatotoxicity. In addition, COQ10 might have stimulated hepatic cell growth, antioxidant regeneration, and inhibited hepatic cell death.[38]
In the current study, supplementation with COQ 10 abrogate FLU-induced hepatotoxicity in albino rats in a dose-dependent fashion. COQ 10 may have clinical application in hepatotoxicity associated with FLU.
The authors appreciate Dr. Obuma Yibala of the Department of Medical Laboratory Sciences, Niger Delta University, Nigeria, for the histological study and also we appreciate Mr, Obi Cosmos of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria, for animal handling.
Subscribe now for latest articles and news.