

Journal of Medical Sciences and Health
DOI: 10.46347/jmsh.2020.v06i03.010
Year: 2020, Volume: 6, Issue: 3, Pages: 58-65
Original Article
Sudhir Bhandari1, Ajit Singh Shaktawat2, Bhoopendra Patel3, Govind Rankawat4, Amit Tak5, Jitendra Kumar Gupta6, Shivankan Kakkar7, Amitabh Dube8
1Sr. Professor, Department of Medicine, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India,
2Principal Specialist, Department of Medicine, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India,
3Assistant Professor, Department of Physiology, Government Medical College, Barmer, Rajasthan, India, 4Senior Professor, Department of Medicine, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India,
5Senior Resident, Department of Physiology, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India,
6Associate Professor, Department of Physiology, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India,
7Senior Demonstrator, Department of Pharmacology, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India, 8Senior Professor, Department of Physiology, S. M. S. Medical College and Attached Hospitals, Jaipur, Rajasthan, India
Address for correspondence:
Bhoopendra Patel, I-204, Teaching Faculty Quarters, Government Medical College, Barmer - 344 001, Rajasthan, India. Phone: +91-8302453274. E-mail: [email protected]
Objective: Antiviral and immunomodulatory properties of antimalarial drugs chloroquine and hydroxychloroquine (HCQ) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been advocated by some researchers for coronavirus disease (COVID)-19 management. Use of these drugs in rheumatological disorders infected with SARS-CoV-2 needs to be elucidated, where complex interaction between the antiviral and immunomodulatory effects and the pathophysiology of rheumatological disorders might provide some important clues for management of COVID-19. The present descriptive observational study was undertaken to evaluate the epidemiological profile of COVID-19 in patients of rheumatological disorders and possible role of HCQ in these patients.
Materials and Methods: The study population consisted of a total of 4100 patients who were reverse– transcriptase polymerase chain reaction positive for SARS-CoV-2, admitted between March 1 and July 10, 2020, in S.M.S. Medical College and attached hospitals, Jaipur, a tertiary care center of Rajasthan, India. These patients were segregated based on the underlying comorbidities to identify those having rheumatological disorders and were further evaluated for treatment history, disease status, outcome, etc.
Results: The prevalence of rheumatological disorders in COVID-19 patients was 0.17% (n=7) that included rheumatoid arthritis (n=4, 0.10%), systematic lupus erythematosus (n=2, 0.05%), and vasculitis (n=1, 0.02%). The mean age was 29.14 years with more females (n=6, 85.71%) affected than males (n=1, 14.29%). Only one (14.29%) mortality was reported among these patients. One patient each on immunosuppressive and steroid treatment developed severe and critical illness and the rest having mild-to-moderate presentation. Only one patient who was on HCQ treatment developed mild illness.
Conclusions: HCQ has some protective role against COVID-19, especially in patients of rheumatological disorders, where a dual benefit might be achieved through reduced COVID-19 severity and remission of rheumatological disorder. The HCQ treatment along with other immunomodulatory therapies may be continued in patients of rheumatological disorders infected with SARS-CoV-2. A large sample and high quality randomized controlled study is required for more conclusive findings.
KEY WORDS:Hydroxychloroquine, rheumatological disorder, coronavirus disease-19, immunomodulatory, antiviral.
The start of 2020 has witnessed one of the worst pandemics in the history of mankind caused by a novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19). The clinical presentation of novel COVID is varied, ranging from mild-to-moderate symptoms of cough, sore throat, headache, rhinorrhea, vomiting and diarrhea, fever, and shortness of breath to signs and symptoms complex of severe pneumonia, acute respiratory distress syndrome, septic shock, and/or multiple organ failure.[1] SARS-CoV-2 emerged from Wuhan, a city of Hubei Province of China, and has swept across 216 countries infecting 14,971,036 people with 618,017 deaths globally as of July 23, 2020.[2]
Even after four phases of systemic lockdown in India, the curve of COVID-19 has been rising with 426,167 active cases and 29,861 deaths as of July 23, 2020. The worst hit states include the state of Maharashtra followed by Tamil Nadu, New Delhi, Karnataka, and Andhra Pradesh.[3,4] Such a huge burden on health- care system predisposes healthcare workers engaged in the management of COVID-19. What makes the situation even worse is that, despite use of personal protective equipment, healthcare workers have contracted COVID-19.[5] The need to evaluate role of hydroxychloroquine (HCQ) in COVID-19 needs special attention in Indian context as this drug has been recommended as prophylactic therapy to all the frontline health workers in India engaged in direct management of COVID-19 and house hold contacts, as per the advisory of National Taskforce for COVID-19 constituted by the Indian Council for Medical Research (ICMR) issued on March 23, 2020.[6] A revised advisory also included other frontline workers at risk of COVID-19 infection such as surveillance workers, paramilitary, and police personnel.[7]
The rapidly spreading COVID-19 pandemic has kick started a search for the possible therapies against this infection. The researchers have made relentless efforts to identify potential pharmacological agents against COVID-19, such as remdesivir, HCQ, chloroquine, and macrolides. Remdesivir, a nucleoside analog prodrug has shown effectiveness against SARS-CoV-2. Research has also indicated chloroquine and HCQ possessing antiviral and immunomodulatory effects.[8-11] Due to aforementioned properties, these drugs have been advocated for the treatment of COVID-19 by some researcher as a legitimate choice. HCQ has been effective against COVID-19 by suppressing the replication of SARS-CoV-2 and its associated inflammatory process.[9,12]
The repurposed drug HCQ was already been prescribed for the treatment of autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis.[13-15] Moreover, use of these drugs in context of the high prevalence of malaria needs special attention. The complex interaction between the antiviral and immunomodulatory effects of chloroquine and the pathophysiology of rheumatological disorders might provide some important clues for possible therapies against SARS- CoV-2. Thus, the present study was undertaken to evaluate the epidemiological profile of COVID-19 in patients of rheumatological disorders and possible role of HCQ in these patients.
The present descriptive observational study was undertaken to evaluate the epidemiological profile of COVID-19 in patients of rheumatological disorders and possible role of HCQ in these patients. The study population consisted of a total of 4100 COVID-19 patients admitted between March 1, 2020, and July 10, 2020, in S.M.S. Medical College and attached hospitals, Jaipur, a tertiary care center of Rajasthan, India. The demographic and patient data were extracted from their medical records, maintaining the anonymity of the patients. The COVID-19 patients were segregated based on the underlying comorbidities to identify those that had any rheumatological disorders to specifically evaluate its epidemiological profile. Patients with rheumatological disorders were further categorized based on the clinical features, other comorbidities, severity, current status, treatment history, and the final outcome. All the patients were reverse–transcriptase polymerase chain reaction positive for SARS-CoV-2 using the nasopharyngeal and oropharyngeal swab samples as per the ICMR guidelines. The data were expressed as numbers and percentage.
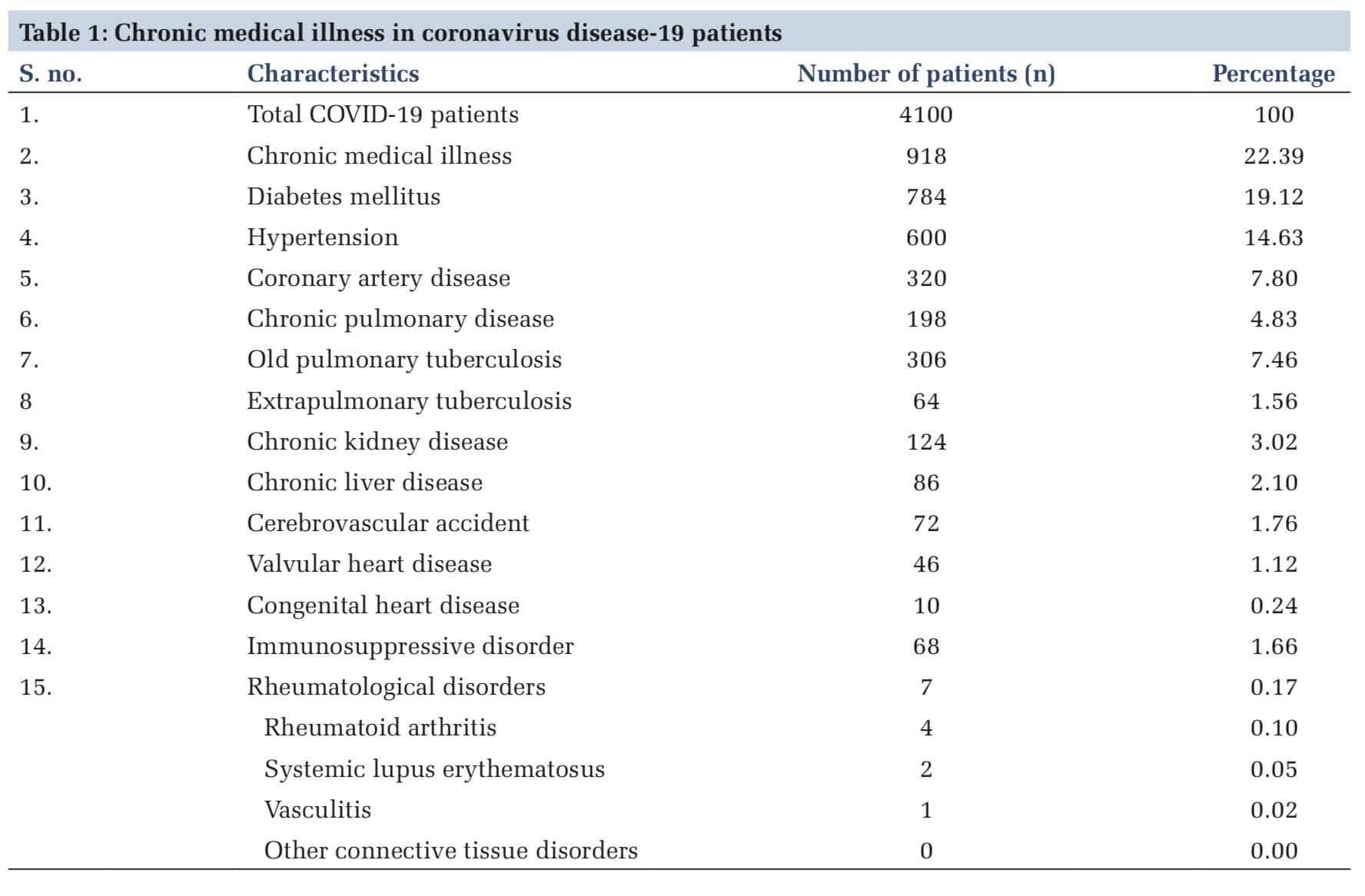
In the present out of 4100 COVID-19 patients admitted, 22.39% (n=918) patients had some chronic medical illnesses. About 19.12% (n=784) patients had diabetes mellitus, 14.63% (n=600) had hypertension, 7.80% (n=320) had coronary heart disease, 7.46% (n=306) had old pulmonary tuberculosis, 4.83% (n=198) had chronic pulmonary disease, 3.02% (n=124) had chronic kidney disease, 2.10% (n=86) had chronic liver disease, and rest had other comorbidities, as shown in Table 1. The prevalence of rheumatological disorders was 0.17% that included rheumatoid arthritis (n=4, 0.10%), systematic lupus erythematosus (n=2, 0.05%), and vasculitis (n=1, 0.02%) [Table 1].
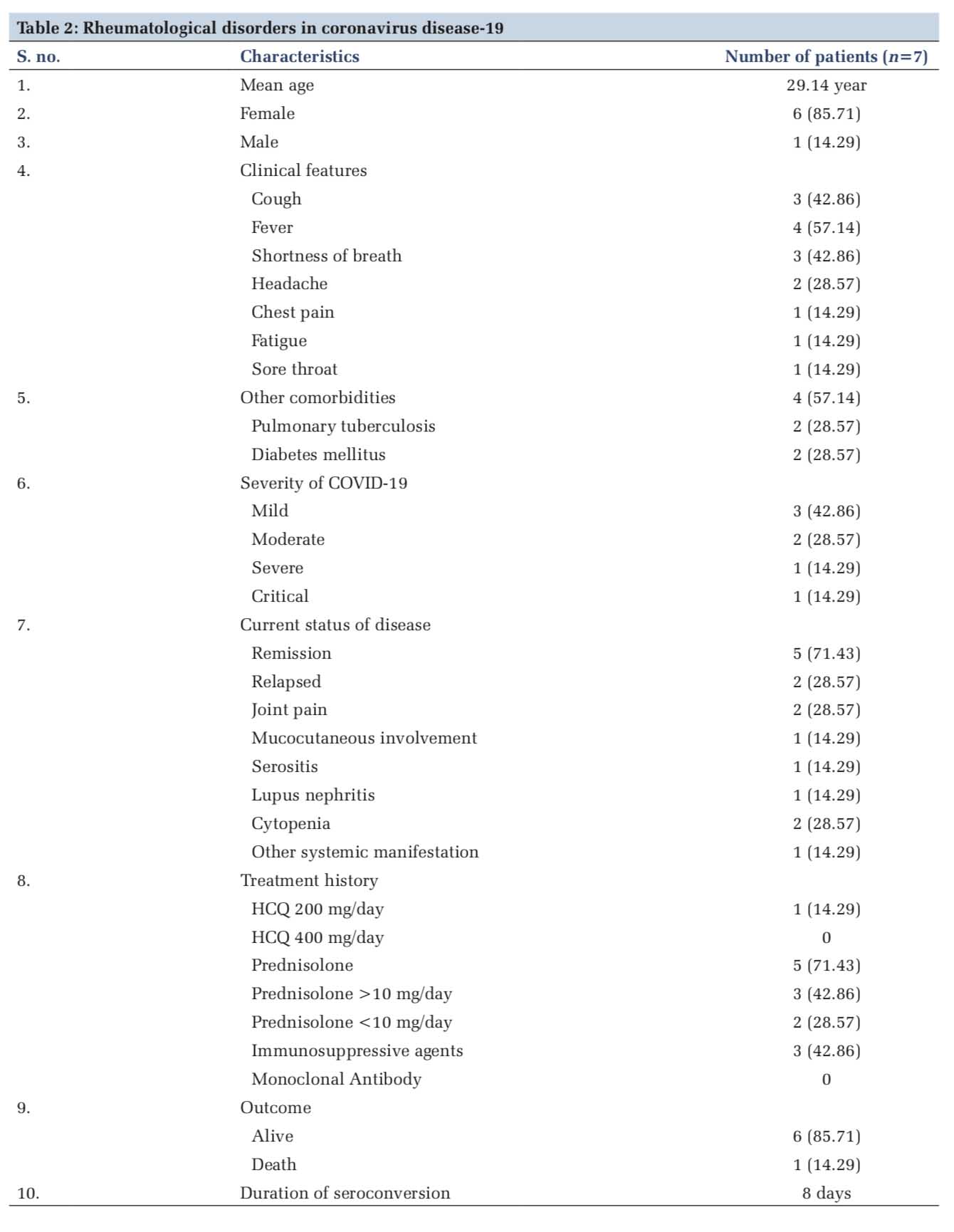
The mean age of COVID-19 patients with rheumatological disorders was 29.14 years. There were more females (n=6, 85.71%) affected than males (n=1, 14.29%). The spectrum of clinical features in these patients included cough (n=3, 42.86%), fever (n=4, 57.14%), shortness of breath (n=3, 42.86%), headache (n=2, 28.57%), chest pain (n=1, 14.29%), fatigue (n=1, 14.29%), and sore throat (n=1, 14.29%). Other comorbidities that were associated include pulmonary tuberculosis (n=2, 28.57%) and diabetes mellitus (n=2, 28.57%). About 42.86% (n=3) patients had a mild illness, 28.57% had moderate illness, and 14.29% (n=1) patients each developed severe and critical illness. About 71.43% (n=5) patients had remission, 28.57% (n=2) had relapse, 28.57% (n=2) had joint pain, 28.57% (n=2) had cytopenia, and 14.29% (n=1) patients each had mucocutaneous involvement, serositis, lupus nephritis, and other systemic manifestation [Table 2].
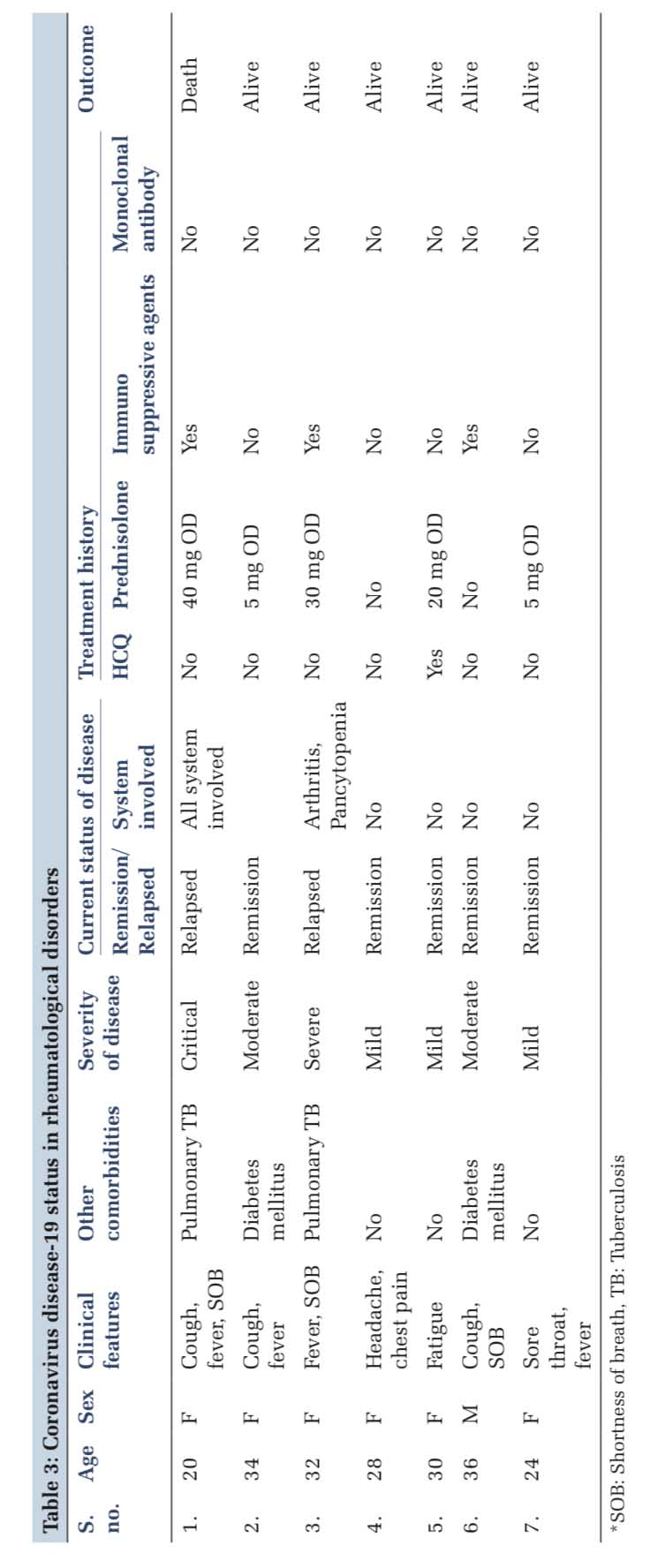
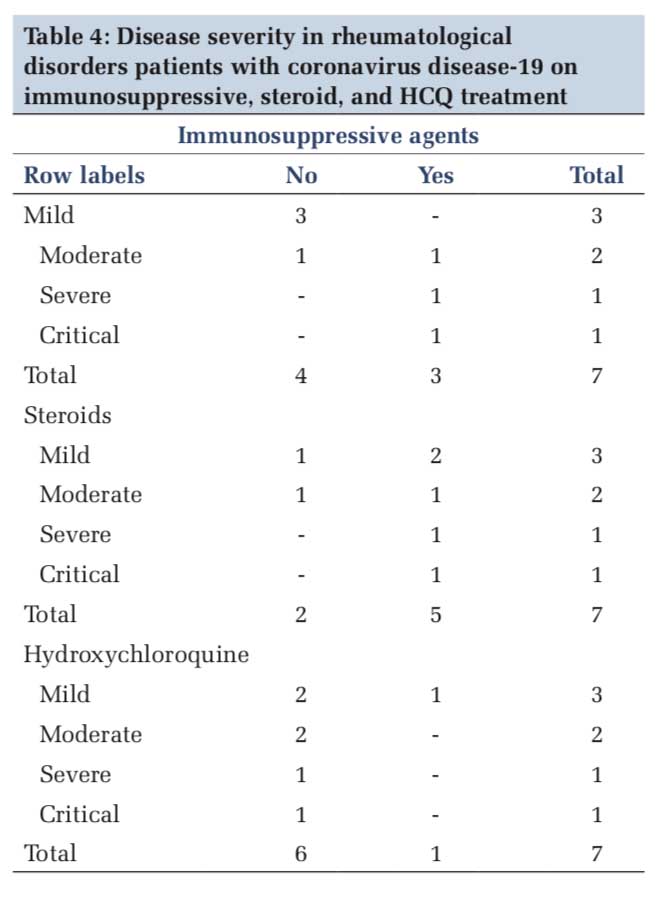
About 14.29% patients were on HCQ 200 mg/day, and no patients were on HCQ 4000 mg/day. Out of 71.43% (n=5) patients who were on prednisolone, 42.86% (n=3) were on prednisolone more than 10 mg/day and 28.57% (n=2) were on prednisolone < 10 mg/day. About 42.86% (n=3) patients were receiving immunosuppressive agents and none was on monoclonal antibodies. Only one (14.29%) mortality was reported among these patients. Duration of seroconversion was 8 days [Tables 2 and 3]. One patient each on immunosuppressive and steroid treatment developed severe and critical illness and the rest having mild-to-moderate presentation. Only one patient who was on HCQ treatment developed mild illness [Table 4].
In the present study, only seven patients with rheumatological disorders were identified positive for SARS-CoV-2 among a study population of 4100patients with only one mortality reported among this group. Only one patient who was on HCQ developed COVID-19 infection with mild symptoms. There could be several patients those might not be infected or if infected were asymptomatic or had mild disease presentation, possibly due to protective effect of HCQ against COVID-19. The debate of using HCQ or not against COVID-19 is important in context of rheumatological disorders as this drug was being prescribed already for rheumatological disorders as a disease-modifying antirheumatic agent (DMARD) for several decades. HCQ has been the mainstay of treatment for rheumatoid arthritis and also effective in other rheumatological disorders combined with physical therapy and aspirin (acetylsalicylic acid) or nonsteroidal anti-inflammatory drugs.[16] The other prominent comorbidities in COVID-19 patients with rheumatological disorders observed in the present study include diabetes mellitus and hypertension that is consistent with the previous research.[17-20]
Research has indicated some role of HCQ against SARS-CoV-2. Immunomodulatory properties along with its ability to interfere with the virus-receptor binding and proliferation might be exploited for COVID-19 management.[21,22] Moreover, it is safer adverse reaction profile than the conventional DMARDs, makes it a preferable choice against COVID-19. However, some serious adverse effects of chloroquine and HCQ, especially cardiac, ophthalmological, and hematological manifestations have hindered its therapeutic use in COVID-19. [23-25] The risk of cardiovascular toxicity is further augmented by its coprescription with drugs such as azithromycin and other comorbid conditions in the form of QTc prolongation and torsades de pointes due to hERG potassium channel blockade.[26,27]
The finding of the present study is consistent with the study in Hubei Province of China that also reported lower risk of SARS-CoV-2 infection in patients with rheumatological disorders on HCQ than other DMARDs.[28] Monti et al. observed that chronic arthritis patients receiving HCQ and infected with SARS-CoV-2 did not develop any severe respiratory complications or mortality.[29] The aforementioned studies indicate some role of HCQ in preventing or reducing the diseases severity of COVID-19 in patients with rheumatological disorders. HCQ is also a widely used drug in cases of SLE and has shown effectiveness against COVID-19 in these patients.[30,31] A study involving 800 SLE patients from rheumatology clinics across India advocated continuing treatment in patients with autoimmune rheumatic diseases. The majority of the patients were on HCQ and a clinically evident cardiac toxicity after its prolonged administration was not reported.[32] Monti et al. also supported the view to continue DMARDs in COVID-19 patients with chronic rheumatological condition.[29]
Angiotensin-Converting Enzyme 2 (ACE2) is the functional receptor for the entry of SARS-CoV-2 in host cells and there are hypomethylation and overexpression of this receptor in SLE patients that can be further aggravated by oxidative stress due to the viral infection. Such an inherent epigenetic dysregulation in lupus might increase the susceptibility of SLE patients to COVID-19 infection, along with a hyper immune response.[33,34] ACE2 receptor must be glycosylated for becoming functional and available for viral entry. HCQ has been shown to inhibit this glycosylation process and hence reduced viral entry into host cells.[35] Thus, the low susceptibility of SLE patients for SARS-CoV-2 could be attributable to the HCQ therapy. HCQ has also been shown to reduce the viral load in COVID-19 patients, especially when used with azithromycin.[36] However, Mathian et al. suggested that HCQ might not protect SLE patients from COVID-19 in its severe form.[37]
One mortality was reported in the present study that could be explained on the basis of severely immunocompromised state of the patients evident from underlying pulmonary tuberculosis, immunosuppressivetherapy,andon40mgprednisolone, possibly leading to severe lung injury, and multiple organ dysfunctions. This patient was not on HCQ treatment.
Patients of COVID-19 may deteriorate rapidly due to the associated cytokine storm.[38] Research has shown role of HCQ in attenuating the pro-inflammatory cytokines, namely, tumor necrosis factor-α, interleukin-1 (IL-1), and IL-1 (IL-6), with a possible role in suppressing the cytokine storm.[39-41] Furthermore, HCQ was also shown to suppress the production of IL-1β and GM-CSF in acute rheumatic fever and IL-6 in patients with rheumatoid arthritis.[42,43] The protective effect of HCQ prophylaxis against COVID-19 is also evident by ability of HCQ in aversion of new infection in South Korea’s long-term care facility, after a large exposure.[44] Moreover, HCQ has shown effectiveness against SARS- COV-2 within safe therapeutic range and still achieving 200–700 times higher concentration in the liver, spleen, kidney, and lung as compared to the plasma.[45,46]
Chatterjee et al. investigated the prophylactic use of HCQ against COVID-19 in healthcare workers and found effectiveness of four or more maintenance doses. Six or more prophylactic doses of HCQ were able to significantly reduce the odds of SARS- CoV-2 infection in healthcare worker.[47] This finding emphasized role of HCQ as prophylactic therapy that could protect against COVID-19, especially against the asymptomatic transmission.
Thus, it can be concluded from the findings of the present study that HCQ has some protective role against COVID-19, especially in patients of rheumatological disorders, where a dual benefit might be achieved through reduced viral load and remission of the disease. COVID-19 patients on HCQ or monoclonal antibodies might be spared from severe outcome of COVID-as compared to those on steroids or other immunosuppressive agents. HCQ may also be playing role in reducing the disease severity. The HCQ treatment along with other immunomodulatory therapies may be continued in patients of rheumatological disorders infected with SARS-CoV-2. However, its use in treatment of severe COVID-19 disease and in patients with known cases of cardiovascular disease needs further validation.
One limitation of this study was that patients of rheumatological disorders who did not contract the COVID-19 and were on HCQ could not be traced. Such a data could provide more conclusive findings regarding effectiveness of HCQ in COVID-19 in rheumatological disorders. Another limitation of this study was its small sample size of the patients with rheumatological disorders. A large sample and high quality randomized controlled trial in future could provide a more conclusive finding regarding the effectiveness of HCQ in these patients.
Subscribe now for latest articles and news.