

Journal of Medical Sciences and Health
Year: 2019, Volume: 5, Issue: 2, Pages: 19-23
Original Article
Rakesh Kumar1, Gurjeet Singh2
1Department of ENT, NC Medical College and Hospital, Panipat, Haryana, India,
2Department of Microbiology, NC Medical College and Hospital, Panipat, Haryana, India
Address for correspondence:
Dr. Gurjeet Singh, Department of Microbiology, NC Medical College and Hospital, Israna, Panipat - 132 107, Haryana, India. E-mail: [email protected]
Background: Chronic suppurative otitis media (CSOM) is an inflammation of the middle ear irrespective of the etiology or pathogenesis. CSOM is a disease of multiple etiologies and is well known for its persistence and recurrence in spite of treatment. It is renowned for its arrival suffering disease. Aim: This study aims to study the bacterial pathogens and their antibiotic sensitivity pattern of ear infections in patients with chronic otitis media.
Materials and Methods:This prospective study was conducted at the Department of ENT and Department of Microbiology, Medical College and Hospital, India, over a period of 1 year from January 2018 to December 2018. A total of 100 patients were included in this study. The ear discharge which is collected with sterile swabs is subjected to Gram’s staining and culture of the causative organism. Antibiotic sensitivity test of cultured bacterial growth is undertaken to know the susceptibility of the causative organism.
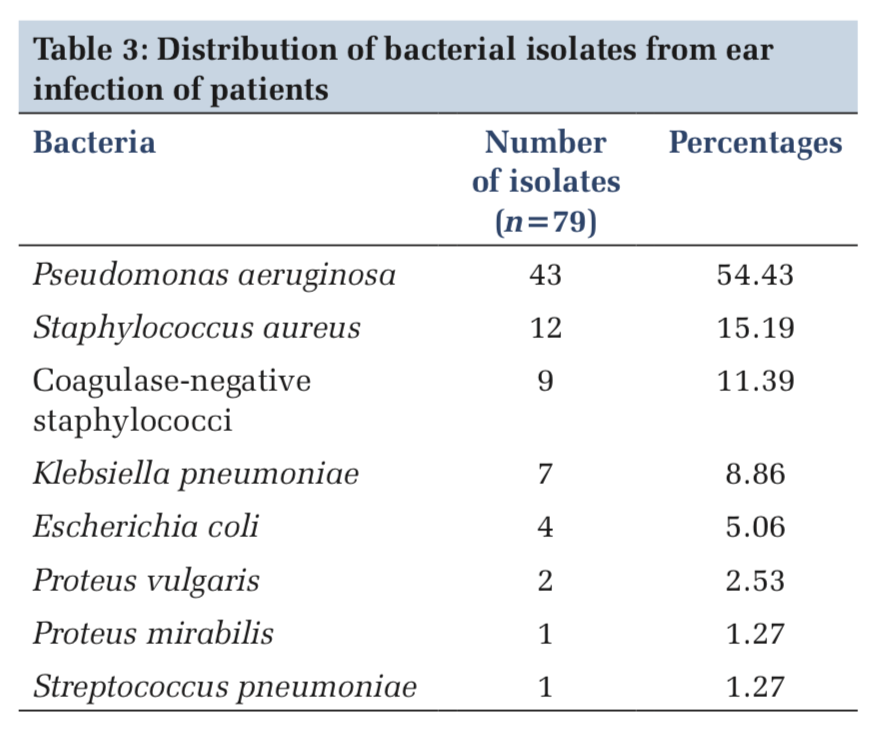
Results: Of 100 samples, 72 were positive for microbial growth and 28 showed no growth. The most common bacteria causing CSOM was Pseudomonas aeruginosa in 43 (54.43%) of samples followed by Staphylococcus aureus 12 (15.19%), coagulase-negative staphylococci 9 (11.39%), Klebsiella pneumoniae 7 (8.86%), Escherichia coli 4 (5.06%), Proteus vulgaris 2 (2.53%), and Proteus mirabilis and Streptococcus pneumoniae 1 (1.27%) each. Susceptibility test was done for known the best antibiotic agents which can be used as a proper treatment to CSOM infection.
Conclusions: In the present study, the most effective antibiotics agents for most of bacterial isolates were gentamicin, ciprofloxacin, amikacin, and chloramphenicol.
KEY WORDS:Antibiotic sensitivity testing, chronic suppurative otitis media, ear infection, pathogenic bacteria.
Chronic suppurative otitis media (CSOM) is an inflammation of the middle ear irrespective of the etiology or pathogenesis. CSOM is disease of multiple etiologies and is well known for its persistence and recurrence in spite of treatment. The causes and pathogenesis of CSOM are multifactorial. CSOM is usually a sequel of acute otitis media.[1] CSOM is a common cause of hearing impairment in developing countries.[2] The infection of the middle ear pursues viral diseases of the upper respiratory tract yet before long attacks the middle ear with pyogenic organisms. The majority of these infections is caused by bacteria. The indiscriminate, imprecise, improper, and haphazard use of antibiotics have caused the emergence of multiple resistant strains of bacteria, which can produce both primary and post-operative infections.[1,3]
The etiology, recurrence, and antimicrobial resistance examples of ear contamination are distinctive in various land territory and atmosphere conditions. As per reports of numerous investigations, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus mirabilis, Klebsiella pneumoniae, and Escherichia coli are the basic life forms disconnected from instances of ear disease. The increased bacterial resistance to many commonly used antibiotics is posing a serious threat to public health. The changes are occurring in the microbiological flora following the advent of sophisticated synthetic antibiotics.[4]
The expanded bacterial protection from numerous ordinarily utilized antibiotics is representing a genuine danger to public health. What’s more, ear disease may prompt tumor in the center ear, post- aural swelling, and aural sinus intricacies. Due to the restricted microbiology research facility setup, doctors in the investigation territory recommend both of the accompanying medications: Amoxicillin, amoxicillin/clavulanic corrosive, chloramphenicol, gentamicin, or ciprofloxacin without the direction of culture and anti-toxin weakness tests to treat patients with possible of otitis media which could prompt rise of medication obstruction. Therefore, data on antibiotic resistance should be accessible at national and neighborhood level to direct the normal utilization of the current antimicrobials.[4]
The aim of this study mainly is to detect the bacterial isolates in patients with otitis media infections and their antibiotics susceptibility pattern against bacterial pathogens among patients attending a tertiary care hospital in Panipat district.
This prospective study was conducted at the Department of ENT and Department of Microbiology, Medical College and Hospital, India, over a period of 1 year from January 2018 to December 2018. The population under study was among people attending the outpatient department of ENT in Medical College and Hospitals, India. A total of 100 patients with signs and symptoms of CSOM who were not on any antibiotics were included and investigated in this study, their ages ranged from 2 to 70 years.
The samples collected from patients with ear exudates using two sterile cotton swabs under strict aseptic precautions and send immediately to microbiology laboratory without delay. The swabs were inoculated onto blood agar, MacConkey agar, and Chocolate agar. The inoculated culture plates were incubated at 37°C for 24 h. Then, a Gram stain examination was made from the second swab of specimen and examined with light microscopy.
Identification of bacterial isolates was done by standard microbiological tests.[5] Furthermore, the antibiotic sensitivity testing of the bacterial isolates was performed by Kirby–Bauer disc diffusion method on Muller-Hinton agar. The result of plates was read after incubation overnight at 37°C by measure of the inhibition zones around the discs of antibiotics as per Clinical Laboratory Standards Institute.[6]
In the present study, of total 100 ear swabs, samples were collected and processed from 100patients suffering from CSOM. Of 100 cases studied, pure growth was obtained in 65 (65%), mixed growth in 7 (7%) while no growth in 28 (28%).
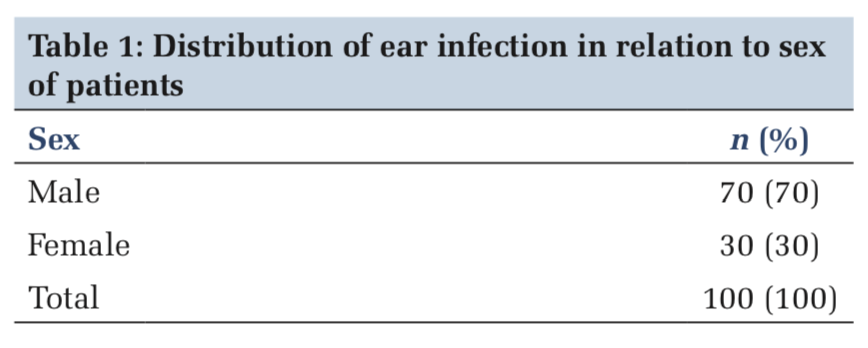
The sex-wise distribution of patients was maximum males (70%) and females (30%). The male-to-female ratio was 3:1 (Table 1).
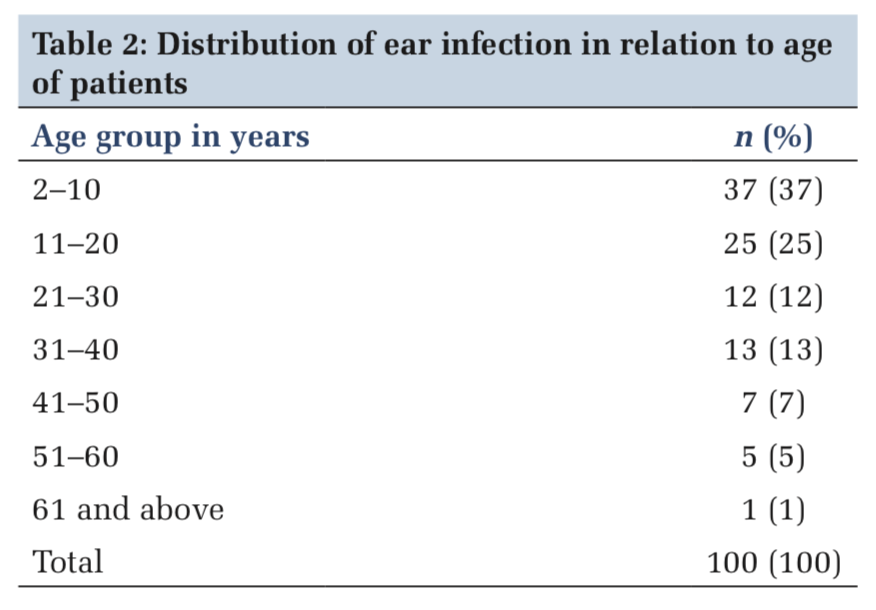
Their ages ranged from < 10 to 61 and above years. The peak incidence of CSOM in age groups 2–10 (37%) followed by age groups 11–20 (25%), 31–40 (13%), 21–30 (12%), 41–50 (7%), 51–60 (5%), and 61 and above (1%) (Table 2).
The most common bacteria causing CSOM was P. aeruginosa in 43 (54.43%) of samples followed by S. aureus 12(15.19%), coagulase-negative staphylococci 9 (11.39%), K. pneumoniae 7 (8.86%), E. coli 4(5.06%), Proteus vulgaris 2(2.53%), and P. mirabilis and Streptococcus pneumoniae 1 (1.27%) each (Table 3).
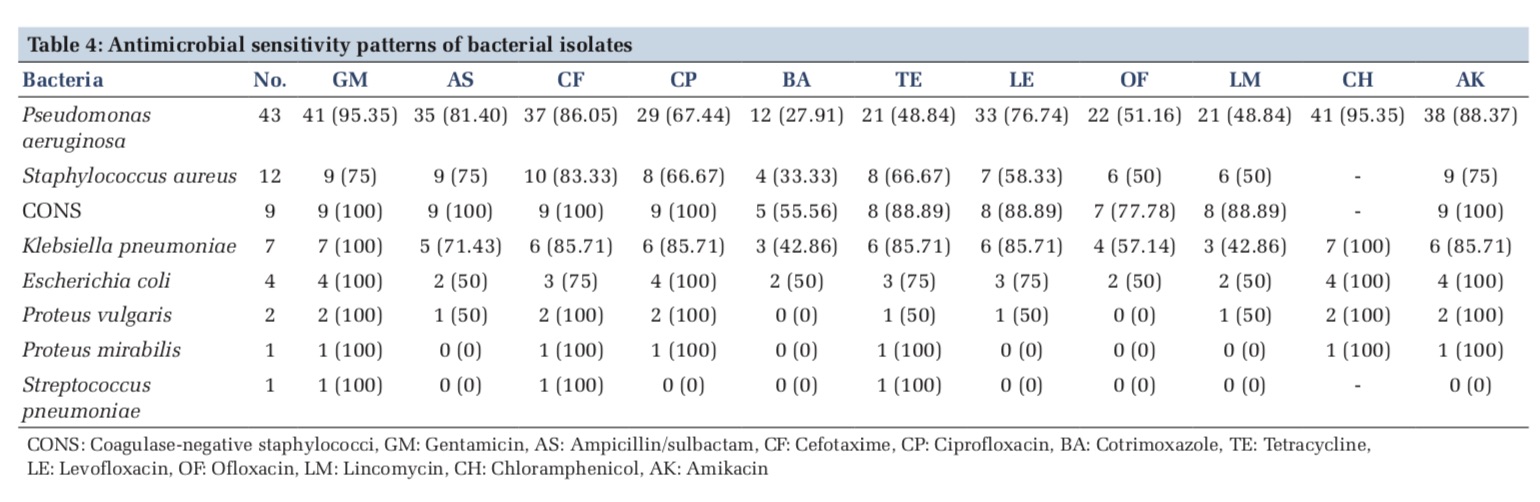
The results of sensitivity testing are described in Table 4. Among the 43 isolates of P. aeruginosa, it was sensitive to gentamicin and chloramphenicol 41 (95.35%) each, amikacin 38 (88.37%), cefotaxime 37 (86.05%), ampicillin/sulbactam 35 (81.40%), levofloxacin 33 (76.74%), ciprofloxacin 29 (67.44%), ofloxacin 22 (51.16%), lincomycin and tetracycline 21 (48.84%) each, and cotrimoxazole 12 (27.91%). S. aureus was maximum sensitive to cefotaxime10 (83.33%), followed by gentamicin, ampicillin/sulbactam and amikacin 9 (75%) each, ciprofloxacin and tetracycline 8 (66.67%) each, levofloxacin 7(58.33%), ofloxacin and lincomycin 6 (50%) each, and cotrimoxazole4 (33.33%). Coagulase-negative staphylococci were maximum sensitive to gentamicin, ampicillin/sulbactam, cefotaxime,ciprofloxacin,andamikacin9 (100%)each, tetracycline, levofloxacin, and lincomycin 8 (88.89%) each, ofloxacin 7 (77.78%), and cotrimoxazole 5 (55.56%). Klebsiella pneumonia was maximum sensitivetogentamicinandchloramphenicol7 (100%), cefotaxime, ciprofloxacin, tetracycline, levofloxacin, and amikacin 6 (85.71%) each, ofloxacin 4 (57.14%), and cotrimoxazole and lincomycin 3 (42.86%). E. coli was sensitive to gentamicin, ciprofloxacin, chloramphenicol, and amikacin 4 (100%), cefotaxime, tetracycline, and levofloxacin 3 (75%) each, and ampicillin/sulbactam, cotrimoxazole, ofloxacin, and lincomycin 2 (50%). P. vulgaris was sensitive to gentamicin, cefotaxime, ciprofloxacin, chloramphenicol, and amikacin 2 (100%), ampicillin/sulbactam, tetracycline, levofloxacin, and lincomycin1(50%), and, however, 100% resistant to ofloxacin and cotrimoxazole. P. mirabilis was sensitive to gentamicin, cefotaxime, ciprofloxacin, tetracycline, chloramphenicol, and amikacin 1 (100%) and, however, 100% resistant to ampicillin/ sulbactam, cotrimoxazole, levofloxacin, ofloxacin, and lincomycin. S. pneumoniae was sensitive to gentamicin, cefotaxime, and tetracycline 1 (100%) each and, however, 100% resistant to ampicillin/ sulbactam, ciprofloxacin, cotrimoxazole, levofloxacin, ofloxacin, lincomycin, and amikacin (Table 4).
The most ailment in oftentimes for patients to visit clinicians with take antimicrobials is ear contamination and the genuine human services concern around the world; it is the otitis media. The incidence of the otitis media in previously publications which reported its depend on the race the socioeconomic factors.[7-13]
CSOM is a condition of the middle ear that is characterized by diligent or intermittent release through an endless aperture of the tympanic layer. Due to puncturing of the tympanic membrane, microorganisms may pick up passage to the center ear by means of the outer ear.[2] Although such serious complications are low at present, still some patients have complications ranging from persistent otorrhea, mastoiditis, labyrinthitis, and facial nerve palsy.[1,9,14-16] Even, however, the complexities are uncommon, treatment ought to be begun early and viably to dodge and lessen the odds of complications.[17] The therapeutic use of antibiotics is usually started empirically before the results of microbiological culture. Selection of any antibiotic is influenced by its efficacy, resistance of bacteria, safety, risk of toxicity, and cost. Information of the neighborhood microorganism design and their antimicrobial affectability is basic to permit successful and cost-saving treatment.[17]
Among 100samples collected from 100patients, 72 samples were bacterial culture positive with a culture positivity of 72%. In studies done by Moorthy et al.,[18] Khanna et al.,[19] Poorey and Lyer,[1] Deb and Ray,[20] and Nikakhlagh et al.,[21] the culture positivity was 69%, 84%, 92%, 53%, and 82%, respectively. In our study, all were monobacterial cultures, whereas in studies done by Moorthy et al.,[18] Khanna et al.,[19] Poorey and Lyer,[1] polymicrobial or mixed cultures were obtained in 65% and 7%, respectively.
In the present study, of 79 bacterial isolates, P. aeruginosa was the predominant bacterium in 43 (54.43%) followed by S. aureus in 12(15.19%), coagulase-negative staphylococci 9(11.39%), K. pneumoniae 7(8.86%), E. coli 4(5.06%), P. vulgaris 2(2.53%), P. mirabilis 1 (1.27%), and S. pneumoniae 1 (1.27%) isolates. Moorthy et al.[18] and Kenna et al.[22] found that Pseudomonas was the predominant organism, i.e. 54% and 67%, respectively, in their study. In a study done by Khanna et al.,[19] the most common bacterial isolate was P. aeruginosa 40.57% followed by S. aureus in 36.23% of cases. Nikakhlagh et al.[21] studied that S. aureus is common isolate in 32.4% followed by 21.69% of P. aeruginosa. Poorey and Lyer[1] observed that Pseudomonas pyocyaneus was the most common organism isolated in 35.2% followed by Klebsiella aerogenes in 25.4%. The observations made from different studies indicate that there can be variation in causative organism based on ethnic, geographic factors. In the present study, gentamicin, ciprofloxacin, amikacin, and chloramphenicol drug have emerged as the most effective antibiotic useful for the patients in our study which is sensitive against more than 85% of P. aeruginosa, S. aureus, and other pathogens. In studies done by Sharma et al.[23] and Poorey and Lyer,[1] amikacin was the most effective drug.
All in all, bacterial ear disease is a noteworthy medical issue in the investigation region. P. aeruginosa, S. aureus, and other Gram-negative bacteria were the overwhelming detaches. Most of the isolates were linked with high levels of resistance against cotrimoxazole, ofloxacin, and ampicillin/sulbactam. In any case, gentamicin, ciprofloxacin, chloramphenicol, and amikacin were effective against the majority of the bacterial isolates. Therefore, it is recommended that treatment of ear infection be done when the causative agents as well as the drug sensitivity patterns are known and properly administered. This will enhance better treatment and reduce the burden of the infection on the patients and in the long term, it may reduce the cost of treatment.
Subscribe now for latest articles and news.